Editorial
| Rev Diabet Stud,
2004,
1(3):108-112 |
DOI 10.1900/RDS.2004.1.108 |
Immunotherapy After Recent-Onset Type 1 Diabetes: Combinatorial Treatment for Achieving Long-Term Remission in Humans?
Damien Bresson, Matthias von Herrath
La Jolla Institute for Allergy and Immunology, Department of Developmental Immunology 3, 10355 Science Center Drive, San Diego, California 92121, USA.
Address correspondence to: Matthias von Herrath, e-mail: matthias@liai.org
Keywords: immunotherapy, anti-CD3, antigen-specific intervention, recent-onset type 1 diabetes, regulatory T cells
Introduction
Insulin-dependent diabetes mellitus (IDDM), or type 1 diabetes mellitus (T1DM), is one of the most common autoimmune diseases. This chronic disorder is thought to be caused by auto-aggressive T lymphocytes that enter the pancreatic islets of Langerhans, where they destroy the insulin-producing β-cells. Activation of such cells is probably multi-factorial involving a genetic predisposition [1], environmental triggers [2] such as viruses and perhaps direct damage to the pancreas itself (pancreatic β-cells), for example, caused by a local pro-inflammatory reaction. Since the β-cell destruction is usually advanced when pre-diabetic human individuals are identified by screening for islet-cell antibodies, one can assume that aggressive responses to more than one islet-antigen will be ongoing during this stage of the disease [3]. It is, therefore, not necessarily practical to attempt to anergize or delete all of the auto-aggressive lymphocytes using direct antigen-specific immunotherapy with all of their cognate antigens. Furthermore, strong forms of non-specific systemic immunosuppression are not usually acceptable, since diabetes frequently affects young individuals, and insulin substitution affords a reasonable quality of life. However, insulin cannot prevent all of the late complications of diabetes, and life expectancy is usually reduced by 10 to 15 years [4]. Therefore, a curative immune-based intervention, with low systemic side-effects, which is able to reverse the course of the disease after recent onset is very desirable. One attractive possibility is the antigen-specific induction of auto-reactive regulatory cells (Treg) that can act as bystander suppressors and simultaneously dampen multiple auto-aggressive T cells with heterogeneous specificities. However, in order to bring such a strategy closer to a potential application in humans, several obstacles still have to be overcome. Indeed, it is not precisely known (i) what effector mechanisms are used by autoreactive regulatory cells; (ii) how to safely choose the appropriate autoantigen suited for antigen-specific immune intervention; or (iii) how to optimize the efficacy of such immunotherapy.
Antigen-specific prevention of type 1 diabetes
Since the early eighties several approaches have been studied for bringing antigen-specific therapy closer to the clinic. Antigen-specific prevention of T1DM can be achieved in several ways in animal models, (i) with DNA vaccines [5-7], (ii) peptide derived from islet antigens [8, 9], or (iii) full-length islet proteins [10, 11] (Table 1). The use of DNA vaccination to prevent T1DM was pioneered by our group, and corroborated and extended by other laboratories. As an example, immunization with a plasmid expressing the insulin B-chain can prevent T1DM in approximately 50% of rat insulin promoter-lymphocytic choriomeningitis virus (RIP-LCMV) mice (virally-induced T1DM model) and NOD mice [6]. Protection is mediated by insulin B-chain specific CD4+ lymphocytes that can protect pre-diabetic recipients upon adoptive transfer. Such auto-reactive CD4+ lymphocytes with beneficial effector functions act in vivo by selectively proliferating in the pancreatic draining lymph node (where insulin is presented as an autoantigen during diabetes development). There, they suppress the expansion and activation of auto-aggressive cells, likely via modulation of antigen-presenting cells [7]. The intriguing aspect about this type of intervention is that it acts antigen-specifically and therefore locally, in this way avoiding systemic side-effects. Protection from diabetes is sustained and does not require repeated antigenic injections. However, most of these antigen-specific immuno-interventions are only protective when administered during the pre-diabetic phase in animal models. Furthermore, even if several different antigen-specific treatments prevent T1DM in animal models (NOD or RIP-LCMV), none of them was able to stop the progression of the disease in humans so far, even when administered before disease onset.
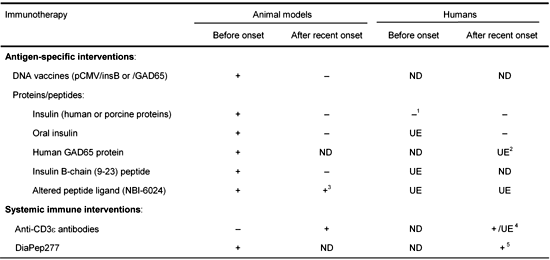
Table
1.
Evaluation of the efficacy of some immunotherapies for treating type 1 diabetes in animal models and humans |
|
 |
 |
Legend:
Successful (+) or unsuccessful (-) immunotherapies are indicated. ND: not determind. UE: under evaluation. 1 Effect in subset of patients. 2 1-year data in patients with latent autoimmune diabetes in adults (LADA) are promising. 3 30% protection compared to the control in the NOD model. 4 Anti-CD3ε treatment after recent-onset showed a positive effect on C-peptide levels over the first year [17]. 5 DiaPep277 treatment after recent-onset diabetes showed a positive effect on C-peptide levels over the first year [13]. |
|
Interestingly, two therapeutic immunizations using peptides are currently under evaluation in clinical trials for treating patients with recent-onset T1DM (Table 1). First, the neurocrine altered-peptide ligand (APL or NBI-6024) is based on the immunodominant T1DM autoantigen insulin B-chain peptide (B:9-23) but is modified with two alanine substitutions at residues 16 and 19. Such a peptide, emulsified in incomplete Freund's adjuvant (IFA), is able to significantly reduce the disease incidence when injected subcutaneously into NOD mice which are overtly diabetic [12]. Secondly, a heat-shock protein (hsp) peptide (DiaPep277) derived from the hsp60 immunodominant epitope within the amino acid residues 437-460 (p277) can preserve endogenous insulin production after a randomized, double-blind, phase II trial in some newly diagnosed T1DM patients [13]. Although the mechanism by which the DiaPep277 induces its 'protective' activity is not fully known, it is thought that hsp60 activates T cells via the Toll-like receptor 2 [14]. This pathway could also be used by the DiaPep277 for inducing the shift from T-helper-1 to T-helper-2 cytokines observed in humans. Thus, this mode of action would categorize DiaPep277 as a systemic immune modulator more than an antigen-specific therapeutic peptide. Whether DiaPep277 induces antigen-specific regulatory cells is unknown. Such evaluation could definitively characterize this peptide as an antigen-specific immunomodulator or not.
Even if antigen-specific based approaches constitute novel and exciting avenues for treating T1DM, there are at least two main issues that require clinical attention: first, not all islet antigens are equally suited for inducing protection; and second, it is conceivable that immunization with an autoantigen might accelerate instead of cure the disease.
Non-mitogenic anti-CD3ε antibody, a powerful tool for blocking recent-onset type 1 diabetes
One of the most promising interventions over the last decade that was initially discovered by the Bach and Chatenoud group and used for the prevention of organ allograft rejection or as treatment of T1DM in the NOD model, and thereafter developed for clinical use by Bluestone and Herold, is the application of non-mitogenic anti-CD3ε antibody (Ab) [15-17]. For mice, this Ab is produced as an engineered F(ab')2 fragment of the hamster anti-CD3ε monoclonal Ab (145 2C11) or for humans as a fully humanized IgG1 (hOKT3gamma1(Ala-Ala)) with a mutated Fc region that prevents it from binding to the Fc receptors, which could trigger undesired effector functions.
Treatment with anti-CD3ε Ab has two important features: first, it is effective in already diabetic NOD mice and can rapidly and very effectively reverse the course of the disease [15]. It is therefore given to recent-onset diabetics in an ongoing clinical trial, where over the first year a positive effect on C-peptide levels was observed, indicating increased preservation of remaining β-cell mass after anti-CD3ε treatment. However, there is now indication that long-term tolerance might not be achieved with a single course of anti-CD3ε, and an increase in the dose could be hazardous, due to side effects such as fever or lymphopenia [17]. Second, as first indicated from studies in the NOD mouse, anti-CD3ε not only affects already committed auto-aggressive effector lymphocytes but also augments regulatory T cells [18, 19]. It is not clear at this point, which phenotype and effector functions these regulators exhibit. As recently demonstrated by Chatenoud et al. [18] in the NOD model, they are CD25+CD62L+ or CD25-CD62L+ (sub-population), and most likely require TGF-β to mediate durable regression of overt diabetes. In humans, CD25+ levels are up-regulated after anti-CD3ε treatment, but, interestingly, ex vivo production of IL-5 and IL-10 is significantly increased in the patients' sera treated with anti-CD3ε (hOKT3gamma1(Ala-Ala)) while augmented levels of TNF-α, IL-2 and IL-6 were also detected [16, 20]. Taken together, all these data argue that anti-CD3ε Ab acts as a systemic immune modulator and induces cytokine-mediated regulation both in the NOD model and humans.
Anti-CD3ε systemic treatment combined with antigen-specific immuno-intervention, a new therapy for long-term remission in humans?
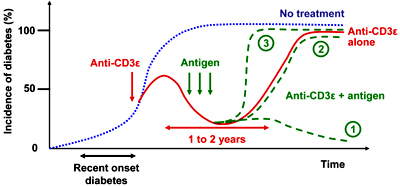
After several years of testing antigen-specific immuno-interventions to treat T1DM in animal models, the general conclusion is that such treatments have to be given early (during the pre-diabetic phase) for maximum efficacy. Thus, these interventions would be suited for a prevention trial but not a recent-onset trial (which would be safer and require a shorter time frame for initial human therapy). Therefore, antigen-specific interventions will need 'help' to be used in human recent-onset diabetics. The fact that anti-CD3ε Ab is able to rapidly 'reset' the immune system during recent-onset diabetes and block the progression of disease in mice makes this Ab a good candidate for opening a therapeutic window for antigen-specific therapy after recent-onset T1DM. This strategy might enable antigen-specific immunizations to induce regulation, such as activation of regulatory T cells, which can maintain long-term tolerance (Figure 1). We would like to point out some important milestones for finding the most potent and safest anti-CD3ε/antigen combination. First, the antigen(s) showing the best synergistic effect with anti-CD3ε treatment should be defined by using at least two different animal models of T1DM, especially since data indicate that prevention/cure of T1DM in animal models is easier than in humans. Therefore, a therapeutic combination restoring euglycemia in several animal models would provide a stronger rationale for a clinical trial in humans. Second, dose and timing of antigen delivery (during or after Ab therapy, or both), as well as the route of administration (intramuscular, subcutaneous, intranasal) have to be evaluated. Third, we have to keep in mind that such a combinatorial treatment is unlikely to be effective once more than 90% of the β-cell mass in humans has been destroyed. Thus, after the clinical onset of T1DM, the characterization of a therapeutic window, where (i) anti-CD3ε will be the most efficient in creating the best conditions for antigen-specific intervention, and (ii) such double therapy remains able to reverse the course of the disease, will be highly desirable.
 |
 |
Figure 1. Schematic representation of the incidence of diabetes after systemic anti-CD3ε treatment in combination with or without antigen-specific intervention. Systemic anti-CD3ε treatment after recent-onset type 1 diabetes is able to reverse the course of the disease, at least partially for 1 to 2 years (red line), opening a window for synergizing with antigen-specific interventions for inducing long-term remission (dashed, green line no. 1). However, antigen-specific interventions with the ‘wrong’ islet antigen could also be without any effect (dashed, green line no. 2), or even accelerate the course of the disease (dashed, green line no. 3). |
|
Finally, the question of how to treat T1DM by immuno-specific interventions in order to obtain long-term tolerance and remission in humans remains unanswered. Combining systemic and antigen- (site-) specific immuno-interventions should increase the efficacy of treatment and will be, in our opinion, the best way to bring antigen-specific interventions to the clinic.
Acknowledgments:
This work is supported by NIH grants AI51973, AI44451 and DK51091 to M.G.V.H.. D.B. is recipient of a fellowship from the European Association for the Study of Diabetes/American Diabetes Association (2003 EASD/ADA Transatlantic Fellowship).
References
- Merriman TR, Todd JA. Genetics of autoimmune disease. Curr Opin Immmunol 1995. 7:786-792. [DOD] [CrossRef]
- Akerblom HK, Vaarala O, Hyoty H, Ilonen J, Knip M. Environmental factors in the etiology of type 1 diabetes. Am J Med Genet 2002. 115:18-29. [DOD] [CrossRef]
- Bach JF, Koutouzov S, van Endert PM. Are there unique autoantigens triggering auto immune diseases? Immunol Rev 1998. 164:139-155. [DOD]
- Liu E, Eisenbarth GS. Type 1A diabetes mellitus-associated autoimmunity. Endocrinol Metab Clin North Am 2002. 31:391-410. [DOD] [CrossRef]
- Coon B, An LL, Whitton JL, von Herrath MG. DNA immunization to prevent autoimmune diabetes. J Clin Invest 1999. 104:189-194. [DOD]
- von Herrath MG. Vaccination to prevent type 1 diabetes. Expert Rev Vaccines 2002. 1:25-28. [DOD] [CrossRef]
- Li AF, Escher A. Intradermal or oral delivery of GAD-encoding genetic vaccines suppresses type 1 diabetes. DNA Cell Biol 2003. 22:227-232. [DOD] [CrossRef]
- Daniel D, Wegmann DR. Protection of non-obese diabetic mice from diabetes by intranasal or subcutaneous administration of insulin peptide B-(9-23). Proc Natl Acad Sci U S A 1996. 93:956-960. [DOD] [CrossRef]
- Martinez NR, Augstein P, Moustakas AK, Papadopoulos GK, Gregori S, Adorini L, Jackson DC, Harrison LC. Disabling an integral CTL epitope allows suppression of autoimmune diabetes by intranasal proinsulin peptide. J Clin Invest 2003. 111:1365-1371. [DOD] [CrossRef]
- Homann D, Dyrberg T, Petersen J, Oldstone MB, von Herrath MG. Insulin in oral immune "tolerance": a one-amino acid change in the B chain makes the difference. J Immunol 1999. 163:1833-1838. [DOD]
- Narendran P, Mannering SI, Harrison LC. Proinsulin-a pathogenic autoantigen in type 1 diabetes. Autoimmun Rev 2003. 2:204-210. [DOD] [CrossRef]
- Alleva DG, Gaur A, Jin L, Wegmann D, Gottlieb PA, Pahuja A, Johnson EB, Motheral T, Putnam A, Crowe PD, et al. Immunological characterization and therapeutic activity of an altered-peptide ligand, NBI-6024, based on the immunodominant type 1 diabetes autoantigen insulin B-chain (9-23) peptide. Diabetes 2002. 51:2126-2134. [DOD]
- Raz I, Elias D, Avron A, Tamir M, Metzger M, Cohen IR. Beta-cell function in new-onset type 1 diabetes and immunomodulation with a heat-shock protein peptide (DiaPep277): a randomised, double-blind, phase II trial. Lancet 2001. 358:1749-1753. [DOD] [CrossRef]
- Zanin-Zhorov A, Nussbaum G, Franitza S, Cohen IR, Lider O. T cells respond to heat shock protein 60 via TLR2: activation of adhesion and inhibition of chemokine receptors. Faseb J 2003. 17:1567-1569. [DOD]
- Chatenoud L, Thervet E, Primo J, Bach JF. Anti-CD3 antibody induces long-term remission of overt autoimmunity in nonobese diabetic mice. Proc Natl Acad Sci U S A 1994. 91:123-127. [DOD]
- Herold KC, Burton JB, Francois F, Poumian-Ruiz E, Glandt M, Bluestone JA. Activation of human T cells by FcR nonbinding anti-CD3 mAb, hOKT3gamma1 (Ala-Ala). J Clin Invest 2003. 111:409-418. [DOD] [CrossRef]
- Herold KC, Hagopian W, Auger JA, Poumian-Ruiz E, Taylor L, Donaldson D, Gitelman SE, Harlan DM, Xu D, Zivin RA, Bluestone JA. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med 2002. 346:1692-1698. [DOD] [CrossRef]
- Belghith M, Bluestone JA, Barriot S, Megret J, Bach JF, Chatenoud L. TGF-beta-dependent mechanisms mediate restoration of self-tolerance induced by antibodies to CD3 in overt autoimmune diabetes. Nat Med 2003. 9:1202-1208. [DOD] [CrossRef]
- Chatenoud L. CD3 antibody treatment stimulates the functional capability of regulatory T cells. Novartis Found Symp 2003. 252:279-290. [DOD]
- Herold KC, Taylor L. Treatment of type 1 diabetes with anti-CD3 monoclonal antibody: induction of immune regulation? Immunol Res 2003. 28:141-150. [DOD]
This article has been cited by other articles:
|