Original Data
| Rev Diabet Stud,
2004,
1(2):89-94 |
DOI 10.1900/RDS.2004.1.89 |
"Low Dose" Metformin Improves Hyperglycemia Better Than Acarbose in Type 2 Diabetics
Ken Yajima1,2, Akira Shimada1, Hiroshi Hirose1, Akira Kasuga3, Takao Saruta1
1Department of Internal Medicine, Keio University School of Medicine, 35 Shinanomachi, Shinjuku-ku, Tokyo, 160-8582, Japan.
2Department of Internal Medicine, Hamamatsu Red Cross Hospital, Hamamatsu, 430-0907, Japan.
3Department of Internal Medicine, Tokyo Denryoku Hospital, Tokyo, 160-0016, Japan.
Address correspondence to: Akira Shimada, e-mail: asmd@st.itc.keio.ac.jp
Keywords: metformin, acarbose, type 2 diabetes, cross-over trial
Abstract
OBJECTIVES: "High dose" metformin therapy (2,550 mg/day) is reported to improve glycemic control in type 2 diabetic patients with obesity (body mass index (BMI) ≥ 30). Some have reported that metformin therapy, even in low doses (500-750 mg/day), improves glycemic control in non-obese type 2 diabetic patients (BMI approximately 25). However, it is unclear whether "low dose" metformin improves glycemic control better than acarbose in non-obese type 2 diabetic patients, which has been shown to improve glycemic control in type 2 diabetes with obesity. METHODS: We randomly divided 22 non-obese type 2 diabetic patients (mean BMI approximately 25) into two groups (A = 11, B = 11). Group A was treated with "low dose" metformin (500-750 mg/day) for 3 months, and switched to acarbose (150-300 mg/day) for another 3 months. Group B was treated with acarbose first, and then switched to "low dose" metformin. RESULTS: "Low dose" metformin significantly decreased the fasting plasma glucose (FPG) and HbA1c level in both groups A and B, whereas acarbose decreased HbA1c levels in group B but not in group A. Overall, "low dose" metformin significantly decreased HbA1c (p=0.0165) levels as compared to acarbose. CONCLUSION: In conclusion, "low dose" metformin therapy improved glycemic control better than acarbose in non-obese diabetics.
Introduction
Insulin, sulfonylureas (SU), alpha-glucosidase inhibitors (αGI), biguanides (BG), thiazolidinediones (TZDs) and phenylalanine derivative have been used for the treatment of type 2 diabetic patients. Because insulin, SU and TZDs often induce weight gain, diabetologists may hesitate to select these drugs as first choice for the treatment of type 2 diabetic patients with obesity. On the other hand, αGI and BG do not usually induce weight gain [1-6]; therefore, they are considered as first line treatment for obese patients. In fact, the United Kingdom Prospective Diabetes Study (UKPDS) demonstrated the usefulness of acarbose (αGI) [7] and metformin (BG) [8] in treating type 2 diabetic patients with obesity.
Probably because the BMI of the enrolled subjects in UKPDS was approximately 30-35, a higher dose of metformin (2,550 mg/day) was administered to treat those patients [8]. Because the existence of insulin resistance is suggested even in non-obese patients, for example with a BMI of 25 [9, 10], metformin therapy can be considered for the treatment of such patients as well. However, it is unclear whether non-obese patients should be treated with the same dosage of metformin as "obese" patients. Reportedly, the incidence of digestive disturbance increased when dosage of metformin was more than 1,000 mg/day [11]. If "low dose" metformin improves glycemic control in type 2 diabetic patients with lesser BMI, compliance of the medication will be greatly improved. Although it has been reported that "low dose" metformin therapy improved glycemic control in type 2 diabetic patients [12], the effect of metformin therapy was not compared with acarbose, which was shown to improve glycemic control in type 2 diabetes with obesity [7] in their study. Moreover, there has been no report so far regarding the comparison between "low dose" metformin and acarbose therapy, except the cross-over study of the two for very short periods (4 weeks) [13]. Therefore, the evaluation of this issue is not yet concluded. In this study, to compare the effect of "low dose" metformin (500-750 mg/day) and acarbose (150-300 mg/day) in non-obese type 2 diabetic patients, we planned the cross-over trial of the two drugs which are not considered to increase body weight in diabetic patients.
Materials and methods (Table 1)
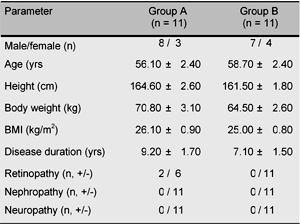
Informed consent was obtained from 22 non-obese type 2 diabetic patients (mean BMI 25.6 ± 0.6), and we randomly divided the patients into two groups (A, B). Most of the patients enrolled in this study were treated without medication. In the case of the patients who were treated with some oral hypoglycemic agents, those drugs were discontinued and a wash-out period of one month was set. Group A (n = 11) was treated with "low dose" metformin (500-750 mg/day) for 3 months, and then switched to acarbose (150-300 mg/day) for another 3 months. Group B (n = 11) was treated with acarbose first, and then switched to metformin. The dosage of metformin was started from 500 mg/day, and 4 weeks later, increased to 750 mg/day, unless the patient complained of symptoms of adverse effects such as digestive disturbance. For the other group, acarbose was started from 150 mg/day, and 4 weeks later, increased to 300 mg/day, unless the patient complained of symptoms of adverse effects such as digestive disturbance. Throughout the study, the patients' diet and exercise therapy were not changed, and no other oral hypoglycemic agent was administered.
As shown in Table 1, age (A: 56.1 ± 2.4 vs. B: 58.7 ± 2.4 years of age), sex (male/female; A: 8/3 vs. B: 7/4), body weight (A: 70.8 ± 3.1 vs. B: 65.4 ± 2.6 kg), BMI (A: 26.1 ± 0.9 vs. B: 25.0 ± 0.8 kg/m2), disease duration (A: 9.2 ± 1.7 vs. B: 7.1 ± 1.5 years), and frequency of diabetic microangiopathy were matched between groups A and B. No patient enrolled in this study had nephropathy. FPG, HbA1c, insulin (IRI), total cholesterol (TC), HDL-cholesterol (HDL-C), triglyceride (TG) levels, and body weight (BMI) were monitored every 3 months.
Table
1.
Baseline characteristics of patients enrolled in this study |
|
 |
 |
Legend:
Data are expressed as mean ± SEM. Data not significant for all parameters. n: number of patients. |
|
At the end of this study, 2 patients in group A took 500 mg/day and 9 patients took 750 mg/day of metformin. All of the patients (n = 11) in group B took 750 mg/day of metformin at the end of the treatment period. On the other hand, 5 patients took 150 mg/day, and 6 patients took 300 mg/day of acarbose in group A, whilst in group B 7 patients took 150 mg/day, and 4 patients took 300 mg/day of acarbose at the end of the study.
All results were expressed as mean ± SEM. A comparison of the mean level was made using student t-test or ANOVA followed by post-hoc Bonferroni/Dunn’s multiple comparison test. The frequency or proportion between the groups was compared using the χ2 test or Fisher's exact test. A P value less than 0.0167 was considered statistically significant in post-hoc Bonferroni/Dunn's multiple comparison test.
Results
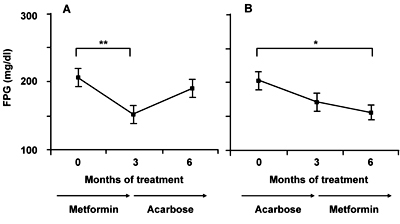
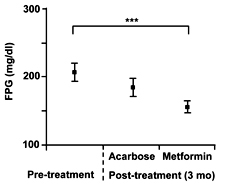
"Low dose" metformin treatment significantly decreased FPG levels in both groups A and B, whereas this was not the case in acarbose treatment (Figure 1). Overall, "low dose" metformin treatment significantly decreased the FPG level as compared to the pretreatment level (p = 0.0001; Figure 2).
 |
 |
Figure 1. Changes in fasting plasma glucose (FPG) level in groups A (A) and B (B). Each value represents mean ± SEM. *p < 0.0167, **p < 0.01. |
|
 |
 |
Figure 2. Changes in fasting plasma glucose (FPG) level after 3-month treatment with acarbose or "low dose" metformin. Each value represents mean ± SEM. ***p < 0.001. |
|
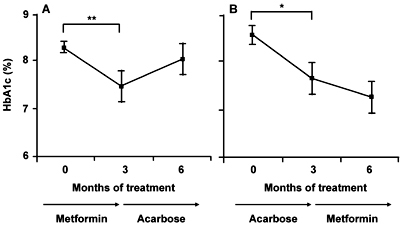
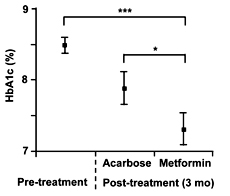
Regarding the HbA1c level, "low dose" metformin treatment for 3 months led to a significant decrease, whereas acarbose rather increased the level after 3 months of treatment in group A. In group B, both acarbose and "low dose" metformin decreased the levels during the 3 months of treatment (Figure 3). Overall, "low dose" metformin significantly decreased the HbA1c level as compared to the pretreatment level (p < 0.0001) and the final level after acarbose treatment (p = 0.0165; Figure 4).
 |
 |
Figure 3. Changes in HbA1c level in groups A (A) and B (B). Each value represents mean ± SEM. *p < 0.0167, **p < 0.01, ***p < 0.001. |
|
 |
 |
Figure 4. Changes Changes in HbA1c level after 3-month treatment with acarbose or "low dose" metformin. Each value represents mean ± SEM. *p < 0.0167, ***p < 0.001. |
|
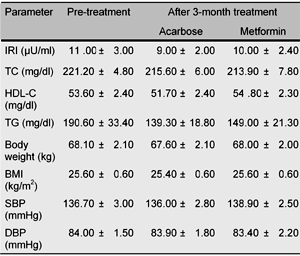
Regarding other parameters such as IRI, TC, HDL-C, TG levels, body weight (BMI), and blood pressure, no significant change was observed (Table 2). Neither metformin nor acarbose induced weight gain in this study.
Table
2.
Changes in various parameters after 3-month treatment with either acarbose or "low dose" metformin |
|
 |
 |
Legend:
Data are expressed as mean ± SEM. |
|
Discussion
The mechanism of improving glucose metabolism with metformin is still unclear, although it has been speculated that the suppression of gluconeogenesis and glucose output in the liver [14], or amelioration of glucose uptake and insulin resistance in the peripheral tissue [6] may contribute. Recently it was reported that metformin activated AMP-activated protein kinase (AMPK), a major cellular regulator of lipid and glucose metabolism, in hepatocytes [15]. There are some reports that metformin ameliorates lipid profiles or blood pressure levels [5, 16-21], although it is not concluded whether the effect depends on glucose metabolism or not. Fatty liver is recognized as one of the features of insulin resistance [22, 23]; metformin may ameliorate insulin resistance by improving fatty liver [24]. Thus, metformin may affect the lipid metabolism independently from glucose metabolism, although metformin does not generally affect lipid parameters and blood pressure.
On the other hand, acarbose delays the digestion of carbohydrate in the small intestine by inhibiting alpha-glucosidase competitively. Therefore, acarbose is usually used to improve postprandial hyperglycemia, although it has been reported that acarbose improved fasting plasma glucose by ameliorating glucose toxicity [7, 25, 26]. Some reported that acarbose ameliorated lipid profiles and blood pressure levels [27-30] by improving glucose metabolism and reducing body weight, although acarbose does not generally affect lipid parameters or blood pressure either.
Although UKPDS indicated that metformin was useful for improving glycemic control in type 2 diabetic patients with obesity, the dosage used in the study was relatively high (2,550 mg/day) and the BMI of the enrolled subjects was about 31. It is unclear whether "high dose" metformin is indeed required to treat type 2 diabetic patients with lesser BMI, i.e. non-obese patients. Reportedly, the frequency of digestive disturbance increased when the dose of metformin exceeded 1,000 mg/day [11]. Therefore, considering the compliance of the medication, confirmation of the finding that "low dose" metformin (500-750 mg/day) was effective in treating non-obese type 2 diabetic patients is clinically important. A previous report showed that "low dose" metformin improved glycemic control as compared to a placebo [11], although it did not improve the pretreatment level of HbA1c. Another report showed that "low dose" metformin monotherapy (or combination therapy with sulfonylurea) was effective in improving glycemic control in type 2 diabetic patients for a period of 6 months [12], although the effect was not compared with αGI. Our present study has shown a new finding, that "low dose" metformin monotherapy improved glycemic control in non-obese type 2 diabetic patients better than acarbose monotherapy. It should be emphasized that metformin action is still operative at the end of the study. This indicates that a terminal has not been attained and the effectiveness of the drug can most probably be prolonged. Although it is unclear why acarbose monotherapy could not improve glycemic control in this population, we speculate that the "lower" dosage (150 mg/day) of acarbose in half of the enrolled patients may partly contribute because of digestive disturbance. Considering the fact that the dosage of metformin in most of the enrolled patients could be increased up to 750 mg/day, "low dose" metformin may also be better than acarbose in the view of the digestive disturbance. Because relatively small numbers of the patients were enrolled in the present study, confirmation with a larger study may be needed in the future.
The 'Diabetes Epidemiology: Collaborative Analysis of Diagnostic Criteria in Europe' (DECODE) study pointed out the importance of postprandial hyperglycemia. Because acarbose is supposed to improve postprandial hyperglycemia in theory, it may be worth trying to combine "low dose" metformin and acarbose to improve the prognosis of non-obese type 2 diabetic patients in a future study as well.
References
- Coniff RF, Shapiro JA, Seaton TB. Long-term efficacy and safety of acarbose in the treatment of obese subjects with non-insulin-dependent diabetes mellitus. Arch Intern Med 1994. 154:2442-2448. [DOD] [CrossRef]
- Feinglos MN, Bethel MA. Oral agent therapy in the treatment of type 2 diabetes. Diabetes Care 1999. 22(Suppl 3):C61-C64. [DOD]
- Wolever TM, Chiasson JL, Josse RG, Hunt JA, Palmason C, Rodger NW, Ross SA, Ryan EA, Tan MH. Small weight loss on long-term acarbose therapy with no change in dietary pattern or nutrient intake of individuals with non-insulin-dependent diabetes. Int J Obes Relat Metab Disord 1997. 21(9):756-763. [DOD] [CrossRef]
- Sangiorgio L, Attardo T, Condorelli L, Lunetta M. Effects of the treatment with acarbose in elderly overweight type 2 diabetic patients in poor glycemic control with oral hypoglycemic agents or insulin. Arch Gerontol Geriatr 2000. 31(1):27-34. [DOD] [CrossRef]
- DeFronzo RA, Goodman AM. Efficacy of metformin in patients with non-insulin-dependent diabetes mellitus. The Multicenter Metformin Study Group. N Engl J Med 1995. 333(9):541-549. [DOD] [CrossRef]
- Davidson MB, Peters AL. An overview of metformin in the treatment of type 2 diabetes mellitus. Am J Med 1997. 102(1):99-110. [DOD] [CrossRef]
- Holman RR, Cull CA, Turner RC. A randomized double-blind trial of acarbose in type 2 diabetes shows improved glycemic control over 3 years (U.K. Prospective Diabetes Study 44). Diabetes Care 1999. 22(6):960-964. [DOD]
- UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998. 352(9131):854-865. [DOD] [CrossRef]
- Espinosa Campos J. Metformin's effects on glucose and lipid metabolism in patients with secondary failure to sulfonylureas. Diabetes Care 1996. 19(11):1185-1189. [DOD]
- Yamakido M. Incidence of non-insulin-dependent diabetes mellitus and its risk factors in Japanese-Americans living in Hawaii and Los Angeles. Diabet Med 1996. 13(9 Suppl 6):S133-S142. [DOD]
- Garber AJ, Duncan TG, Goodman AM, Mills DJ, Rohlf JL. Efficacy of metformin in type II diabetes: results of a double-blind, placebo-controlled, dose-response trial. Am J Med 1997. 103(6):491-497. [DOD] [CrossRef]
- Ohmura C, Tanaka Y, Mitsuhashi N, Atsumi Y, Matsuoka K, Onuma T, Kawamori R. Efficacy of low-dose metformin in Japanese patients with type 2 diabetes mellitus. Current therapeutic research 1998. 59(12):889-895. [DOD] [CrossRef]
- Johansen K. Acarbose treatment of sulfonylurea-treated non-insulin dependent diabetics. A double-blind cross-over comparison of an alpha-glucosidase inhibitor with metformin. Diabete Metab 1984. 10(4):219-223. [DOD]
- Inzucchi SE, Maggs DG, Spollett GR, Page SL, Rife FS, Walton V, Shulman GI. Efficacy and metabolic effects of metformin and troglitazone in type II diabetes mellitus. N Engl J Med 1998. 338(13):867-872. [DOD] [CrossRef]
- Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 2001. 108(8):1167-1174. [DOD] [CrossRef]
- Giugliano D, De Rosa N, Di Maro G, Marfella R, Acampora R, Buoninconti R, D'Onofrio F. Metformin improves glucose, lipid metabolism, and reduces blood pressure in hypertensive, obese women. Diabetes Care 1993. 16(10):1387-1390. [DOD]
- Giugliano D, Quatraro A, Consoli G, Minei A, Ceriello A, De Rosa N, D'Onofrio F. Metformin for obese, insulin-treated diabetic patients: improvement in glycaemic control and reduction of metabolic risk factors. Eur J Clin Pharmacol 1993. 44(2):107-112. [DOD] [CrossRef]
- Vigneri R, Goldfine ID. Role of metformin in treatment of diabetes mellitus. Diabetes Care 1987. 10(1):118-122. [DOD]
- Fedele D, Tiengo A, Nosadini R, Marchiori E, Briani G, Garotti MC, Muggeo M. Hypolipidemic effects of metformin in hyperprebetalipoproteinemia. Diabete Metab 1976. 2(3):127-133. [DOD]
- Rains SG, Wilson GA, Richmond W, Elkeles RS. The effect of glibenclamide and metformin on serum lipoproteins in type 2 diabetes. Diabet Med 1988. 5(7):653-658. [DOD]
- Nagi DK, Yudkin JS. Effects of metformin on insulin resistance, risk factors for cardiovascular disease, and plasminogen activator inhibitor in NIDDM subjects. A study of two ethnic groups. Diabetes Care 1993. 16(4):621-629. [DOD]
- Marchesini G, Brizi M, Bianchi G, Tomassetti S, Zoli M, Melchionda N. Metformin in non-alcoholic steatohepatitis. Lancet 2001. 358(9285):893-894. [DOD] [CrossRef]
- Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, McCullough AJ, Natale S, Forlani G, Melchionda N. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes 2001. 50(8):1844-1850. [DOD]
- Marchesini G, Brizi M, Morselli Labate AM, Bianchi G, Bugianesi E, McCullough AJ, Forlani G, Melchionda N. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med 1999. 107(5):450-455. [DOD] [CrossRef]
- Mertes G. Safety and efficacy of acarbose in the treatment of Type 2 diabetes: data from a 5-year surveillance study. Diabetes Res Clin Pract 2001. 52(3):193-204. [DOD] [CrossRef]
- Chiasson JL, Josse RG, Hunt JA, Palmason C, Rodger NW, Ross SA, Ryan EA, Tan MH, Wolever TM. The efficacy of acarbose in the treatment of patients with non-insulin-dependent diabetes mellitus. A multicenter controlled clinical trial. Ann Intern Med 1994. 121(12):928-935. [DOD]
- Reaven GM, Lardinois CK, Greenfield MS, Schwartz HC, Vreman HJ. Effect of acarbose on carbohydrate and lipid metabolism in NIDDM patients poorly controlled by sulfonylureas. Diabetes Care 1990. 13(Suppl 3):32-36. [DOD]
- Yoshino G, Furukawa S, Hirano T, Naito H, Kazumi T, Urayama T. The minimum dose of acarbose suppresses triglyceride concentration in remnant-like particles from fasted diabetic subjects. Horm Metab Res 1996. 28(7):329-330. [DOD]
- Leonhardt W, Hanefeld M, Fischer S, Schulze J. Efficacy of alpha-glucosidase inhibitors on lipids in NIDDM subjects with moderate hyperlipidaemia. Eur J Clin Invest 1994. 24(Suppl 3):45-49. [DOD]
- Calle Pascual A, Garcia Honduvilla J, Martin Alvarez PJ, Calle JR, Maranes JP. Influence of 16-week monotherapy with acarbose on cardiovascular risk factors in obese subjects with non-insulin-dependent diabetes mellitus: a controlled, double-blind comparison study with placebo. Diabetes Metab 1996. 22(3):201-202. [DOD]
This article has been cited by other articles:
|