Original Data
| Rev Diabet Stud,
2008,
5(4):232-244 |
DOI 10.1900/RDS.2008.5.232 |
Dose- and Glucose-Dependent Effects of Amino Acids on Insulin Secretion from Isolated Mouse Islets and Clonal INS-1E Beta-Cells
Zhenping Liu1,2, Per B. Jeppesen1, Søren Gregersen1, Xiaoping Chen1, Kjeld Hermansen1
1Department of Endocrinology and Metabolism C, Aarhus University Hospital, Aarhus Sygehus THG, Tage-Hansens Gade 2, DK-8000 Aarhus C, Denmark
2The Second Affiliated Hospital of PLA General Hospital, Beijing 100091, P. R. China
Address correspondence to: Kjeld Hermansen, e-mail: kjeld.hermansen@dadlnet.dk
Manuscript submitted January 15, 2009; resubmitted February 16, 2009; accepted February 17, 2009.
Keywords: diabetes, INS-1E beta-cells, amino acid, glucose, insulin secretion
Abstract
BACKGROUND: The influence of glucose and fatty acids on beta-cell function is well established whereas little is known about the role of amino acids (AAs). METHODS: Islets isolated from NMRI mice were incubated overnight. After preincubation, isolated islets as well as clonal INS-1E beta-cells were incubated for 60 min in a modified Krebs Ringer buffer containing glucose and AAs. RESULTS: At 16.7 mmol/l (mM) glucose, L-arginine, L-lysine, L-alanine, L-proline, L-leucine, and L-glutamine potentiated glucose-stimulated insulin secretion dose-dependently, while DL-homocysteine inhibited insulin secretion. Maximal insulin stimulation was obtained at 20 mM L-proline, L-lysine, L-alanine, L-arginine (islets: 2.5 to 6.7 fold increase; INS-1E cells: 1.6 to 2.2 fold increase). L-glutamine and L-leucine only increased glucose-stimulated (16.7 mM) insulin secretion (INS-1E cells: 1.5 and 1.3 fold, respectively) at an AA concentration of 20 mM. Homocysteine inhibited insulin secretion both at 5.6 mM and 16.7 mM glucose. At glucose levels ranging from 1.1 to 25 mM, the equimolar concentration of 10 mM, L-proline, L-lysine, L-arginine increased insulin secretion from mouse islets and INS-1E cells at all glucose levels applied, with a maximal effect obtained at 25 mM glucose. At a concentration of 10 mM, L-arginine and L-lysine had the highest insulinotropic potency among the AAs investigated. CONCLUSION: L-arginine, L-lysine, L-alanine, L-proline, L-leucine and L-glutamine acutely stimulate insulin secretion from mouse islets and INS-1E cells in a dose- and glucose-dependent manner, whereas DL-homocysteine inhibits insulin release.
Introduction
Pancreatic beta-cells play a key role in metabolic control and exquisitely link nutrient sensing to insulin secretion. Glucose homeostasis requires precise control of insulin secretion. This control is primarily ensured by glucose itself, but involves other nutrients and numerous neural and hormonal factors. Thus specific amino acids (AAs) may acutely and/or chronically regulate insulin secretion from pancreatic beta-cells in vivo and in vitro.
Few studies have attempted to explore the interplay between glucose, AAs, and fatty acids with respect to the functional integrity of beta-cells in vitro. The present concept is that AAs stimulate insulin release either through their capacity to act as nutrients in islet cells or, in the case of cationic AAs (e.g. L-arginine), as a result of their accumulation inside the insulin-producing cells of the endocrine pancreas [1]. AAs represent an important class of nutrients which may be grouped according to electrical and metabolic aspects and also by the transport systems involved in their uptake [2].
Studies have indicated that, like many other cell types, the pancreatic beta-cell expresses a number of distinct AA transport systems [2]. Among them, the most important are the so-called systems A, ASC, L, and Gly, responsible for the transport of a wide range of AAs, including L-alanine, L-arginine, L-glutamine, L-leucine, L-lysine, L-proline and L-serine. These AAs share a number of transport systems and are rapidly accumulated within beta-cells. The stimulatory action of AAs on insulin secretion can largely be attributed to three possible mechanisms: metabolism with the generation of ATP (e.g. L-alanine [3], L-leucine [4], L-proline [1]), uptake of cationic AAs resulting in membrane depolarization (L-arginine and L-lysine [5]), and depolarization of membranes through co-transport with Na+ (L-proline and L-alanine [6]). A number of experiments have studied transport, degradation and metabolism of AAs in islet cells [1, 7], but there is further need to clarify the actions of many AAs on the pancreatic beta-cell. There are still conflicting results. For example, regarding alanine-induced insulin secretion, no comparisons of the potency of different AAs in INS-1E cells and isolated mouse islets have been carried out until now. In addition, few studies have investigated the impact of homocysteine on beta-cell function, which is of relevance since the level of this AA is elevated in subjects with the metabolic syndrome [8].
This is the first study testing the acute dose-response relationship and glucose-dependency of a range of AAs on the clonal glucose-sensitive INS-1E beta-cell line and comparing these effects with results obtained in isolated mouse islets.
Materials and methods
Cell culture
Prior to this study, the INS-1E cells (kindly provided by Professor C. B. Wollheim, Geneva, Switzerland) were maintained under culture condition, as described below, and the cells were passaged weekly [9]. The approximate doubling time for these cells was 2-3 d. INS-1E cells were grown in a humidified atmosphere (5% CO2, 95% air at 37°C) in monolayer in modified RPMI 1640 medium supplemented with 10% (v/v) FCS, 11.1 mmol/l (mM) glucose, 10 mM HEPES, 100 IU/ml penicillin, 100 μg/ml streptomycin, 1 mM sodium pyruvate, 50 μM β-mercaptoethanol (all from Gibco BRL, Paisley, UK) and 0.5% BSA (fatty acid free; Roche Molecular Biochemicals, Mannheim, Germany). The passage numbers of INS-1E cells used in this study was 59-71. Simultaneously, some cells were cultured for 48 h in 24-well plates (NUNC Brand Products, NUNC A/S, Roskilde, Denmark) at a density of 2.5 × 105 cells per well for secretion studies.
Insulin secretion study
Cells were preincubated in a modified Krebs-Ringer buffer (KRB) supplemented with 0.1% BSA at 37°C for 30 min. The KRB contained 125 mM NaCl, 5.9 mM KCI, 1.2 mM MgCl2, 1.28 mM CaCl2, and 25 mM HEPES (pH 7.4; all Sigma). The first part of the study was designed to determine the dose-relationship of the AA-mediated insulin secretion from INS-1E cells. After preincubation, cells were placed in 1 ml KRB with the AAs and glucose according to the protocol for 60 min.
The second part of the study was designed to determine the glucose-dependency of the AA-induced insulin secretion from INS-1E cells. After preincubation, cells were placed in 1 ml KRB with 10 mM AAs and different concentrations of glucose according to the protocol for 60 min. After incubation, 300 μl medium was collected and centrifuged, and 200 μl supernatant was stored at -20°C for insulin assay.
Protein assay
The remaining medium was discarded after collecting samples for insulin assay. Cells were washed with cold phosphate buffered saline and lysed in 0.1 mol/l NaOH; the total protein was measured by a detergent-compatible protein kit (Bio-Rad Laboratories, Inc., Hercules, CA).
Experimental animals
Adult female NMRI mice (Taconic, Lille Skensved, Denmark) weighing 20-25 g were used. Animals were fed a standard pellet diet (Altromin, Brogaarden, Gentofte, Denmark) with tap water ad libitum before experiments. The light/dark cycle was 12/12 h. The study was carried out in accordance with the guidelines of the Danish Council on Animal Care.
Isolation and culture of islets
Islets were isolated by the collagenase digestion technique [10]. In brief, the animals were anesthetized with pentobarbital (50 mg/kg) intraperitoneally and a midline laparotomy was made. The pancreas was filled retrogradely with 3 ml ice-cold HBSS (Sigma Chemical, St. Louis, MO, USA) supplemented with 0.3 mg/ml collagenase P (Boehringer Mannheim GmbH, Mannheim, Germany). HBSS and collagenase P were filter sterilized before use. Subsequently, the pancreas was removed and incubated for 19 min at 37°C in a water bath. After rinsing with ice-cold HBSS, the islets were handpicked under a stereomicroscope and incubated overnight at 37°C and 5% CO2/95% normal atmosphere in 10 ml RPMI 1640. The latter contained 11.1 mM glucose supplemented with 10% FCS, 100 IU/ml penicillin G, 100 μg/ml streptomycin (all GIBCO BRL, Paisley, UK). Only medium-sized islets were used. Islets were obtained from 6 to 10 mice to compensate for inter-individual differences.
Insulin secretion studies
After overnight incubation, islets were rinsed once with modified KRB supplemented with 3.3 mM glucose and 0.1% human serum albumin (Sigma, St. Louis, MO, USA). The first part of the study was designed to determine the dose-response relationship of the AA-mediated insulin secretion from mouse islets. After 30 min preincubation at 37°C, single islets were handpicked and incubated in 100 μl KRB with the different AAs and glucose according to the protocol for 60 min. The second part of the study was designed to determine the glucose-dependency of AA-induced insulin secretion from mouse islets. After a 30 min preincubation at 37°C, single islets were handpicked and incubated in 100 μl KRB with 10 mM of the AAs and different glucose concentrations according to the protocol for 60 min. After a 60 min incubation in a normal atmosphere at 37°C, 50 μl of the medium was collected and frozen for insulin analysis.
Insulin assay
Insulin was measured by RIA with a guinea pig, anti-porcine insulin Ab (Novo Nordisk, Bagsvaerd, Denmark), mono-125I-(Tyr-A14)-labeled human insulin (Novo Nordisk A/S) as tracer, and rat insulin as standard (Novo Nordisk). Free and bound radioactivity were separated using ethanol [11]. The inter- and intra-assay variation coefficients were both less than 5%.
Statistical analysis
Data are presented as the mean ± SEM. One-way ANOVA was used for the statistical analysis. Data from each treatment condition was compared with control values. Significant differences between two groups were considered for p < 0.05.
Results
Effects of AAs on glucose-induced insulin secretion from isolated mouse islets and clonal INS-1E cells
In isolated mouse islets, all AAs at concentrations ranging from 0.1 to 20 mM were tested in the presence of 16.7 mM glucose. L-alanine, at concentrations ranging from 0.1 to 20 mM, significantly increased the glucose-induced insulin release by 1.7 to 3.7 fold compared to control (Figure 1A). L-arginine, L-lysine and L-leucine, at concentrations between 5 to 20 mM, stimulated the glucose-induced insulin secretion by 1.6 to 6.7 fold compared to control (Figures 1B, 1C and 1F). L-proline from 10 to 20 mM enhanced the glucose-induced insulin secretion by 1.9 to 2.5 fold compared to control (Figure 1D), and 20 mM L-glutamine increased the glucose-induced insulin secretion by 2.1 fold (Figure 1E).
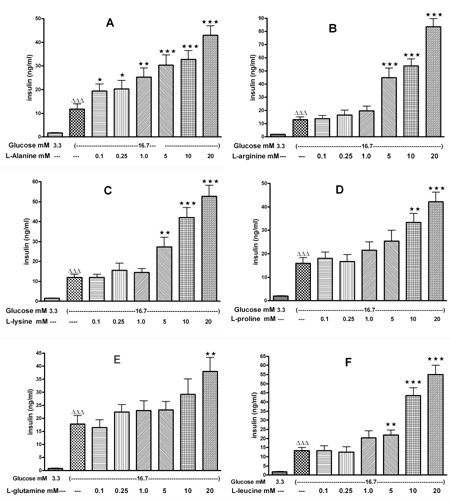 |
 |
Figure 1. The effect of 0.1-20 mM L-alanine (A), L-arginine (B), L-lysine (C), L-proline (D), L-glutamine (E), L-leucine (F) on insulin secretion from mouse islets. Following a 30 min preincubation, we tested the effect of a 60-min incubation with AAs on insulin secretion. Values are means ± SEM (n = 24). ∆ p < 0.05, ∆∆ p < 0.01, ∆∆∆ p < 0.001 comparing the effect at 3.3 mM versus 16.7 mM glucose in the absence of AAs. * p < 0.05, ** p < 0.01, *** p < 0.001 comparing the effect of different concentrations of AAs in the presence of 16.7 mM glucose versus 16.7 mM glucose alone. |
|
In INS-1E cells, the effects of AAs at concentrations ranging from 0.1 to 20 mM were tested in the presence of 16.7 mM glucose. L-alanine (0.25-20 mM) significantly increased the glucose-induced insulin release by 1.2 to 2.2 fold compared to control (Figure 2A). L-arginine (1.0-20 mM) enhanced the glucose-induced insulin secretion by 1.3 to 2.2 fold compared to control (Figure 2B). L-lysine and L-proline (0.1-20 mM) stimulated the glucose-induced insulin secretion by 1.1 to 1.6 fold compared to control (Figures 2C and 2D). L-glutamine and L-leucine, at only 20 mM, increased the glucose-induced (16.7 mM) insulin secretion by 1.5 and 1.3 fold, respectively (Figures 2E and 2F).
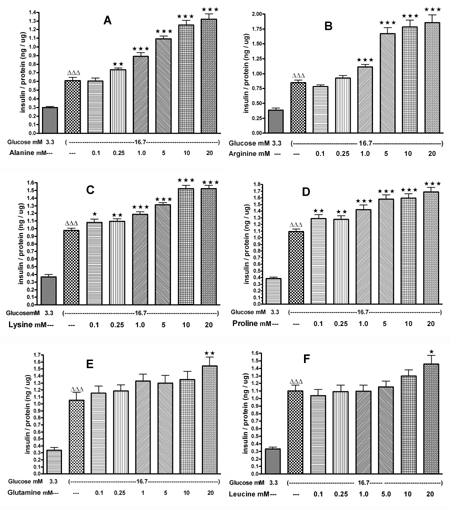 |
 |
Figure 2. The effects of 0.1-20 mM L-alanine (A), L-arginine (B), L-lysine (C), L-proline (D), L-glutamine (E), L-leucine (F) on insulin secretion from INS-1E cells. Following 30 min of preincubation, we tested the effect of a 60-min incubation with AAs on insulin secretion. Values are means ± SEM (n = 24). ∆ p < 0.05, ∆∆ p < 0.01, ∆∆∆ p < 0.001 comparing the effect of 3.3 mM versus 16.7 mM glucose in the absence of AA. * p < 0.05, ** p < 0.01, *** p < 0.001 comparing the effect of different concentrations of AAs in the presence of 16.7 mM glucose versus 16.7 mM glucose alone. |
|
At 5.6 and 16.7 mM glucose, we tested the effect of DL-homocysteine (12.5 to 1000 μM) on mouse islets or INS-1E cells. DL-homocysteine at 1000 μM significantly inhibited insulin secretion, in the presence of 5.6 or 16.7 mM glucose, from mouse islets as well as from INS-1E cells, respectively (Figures 3A-D).
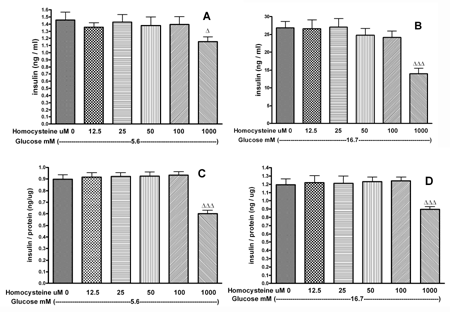 |
 |
Figure 3. The effect of 12.5 μM - 1 mM DL-homocysteine on insulin secretion from mouse islets (A and B) and INS-1E cells (C and D). Following a 30 min preincubation, we tested the effect of a 60-min incubation with homocysteine on insulin secretion. Values are means ± SEM (n = 32). ∆ p < 0.05, ∆∆ p < 0.01, ∆∆∆ p < 0.001 comparing the effect of different concentrations of DL-homocysteine in the presence of 5.6 mM or 16.7 mM glucose versus equivalent glucose alone. |
|
Effects of glucose on AA-induced insulin secretion in islets and INS-1E cells
As shown in Figure 4, we compared the effect of the equimolar concentration of 10 mM L-alanine, L-arginine, L-lysine, L-proline, L-leucine and L-glutamine on insulin secretion from mouse islets in the presence of glucose concentrations between 1.1-25 mM. Even at the lowest glucose level of 1.1 mM, these AAs, with the exception of L-alanine and L-glutamine, increased insulin secretion significantly compared to control (Figures 4A-F). At 3.3 mM glucose, all of these AAs, with the exception of L-alanine, enhanced insulin secretion by 1.2 to 2.7 fold compared to control. At 6.7 or 11.1 mM glucose, all tested AAs significantly increased insulin secretion compared to control. At the highest glucose levels of 16.7 and 25 mM, all AAs, with the exception of L-glutamine, stimulated insulin release by 1.6 to 2.5 fold compared to control (Figure 4).
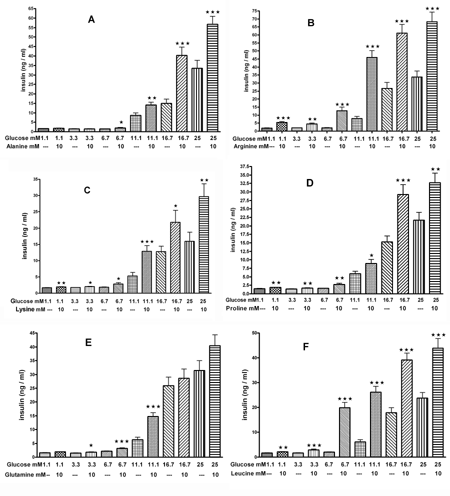 |
 |
Figure 4. The effect of glucose on AA-mediated (10 mM) insulin secretion from mouse islets. A: L-alanine, B: L-arginine, C: L-lysine, D: L-proline, E: L-glutamine, F: L-leucine. Following a 30 min preincubation, we tested the effect of a 60-min incubation with AAs and glucose on insulin secretion. Values are means ± SEM (n = 24). * p < 0.05, ** p < 0.01, *** p < 0.001 comparing the effect of glucose in the presence or absence of 10 mM AAs. |
|
Figure 5 illustrates the insulin response from clonal INS-1E cells to 10 mM of L-alanine, L-arginine, L-lysine, L-proline, L-leucine, and L-glutamine, respectively, in the presence of glucose at concentrations between 1.1 and 25 mM. Even at the lowest glucose concentration (1.1 mM), these AAs, with the exception of L-alanine and L-glutamine, increased insulin secretion compared to control (Figures 5A-F). At 3.3 mM glucose, all tested AAs, with the exception of L-alanine, enhanced insulin secretion by 1.3 to 3.1 fold compared to control. At 6.7 mM glucose, all tested AAs, but L-glutamine, increased insulin release by 1.4 fold compared to control. At the higher glucose levels of 11.1 mM, 16.7 mM, or 25 mM, all tested AAs, but L-glutamine and L-leucine, significantly enhanced insulin release by 1.3 to 1.5 fold compared to control (Figures 5A-F).
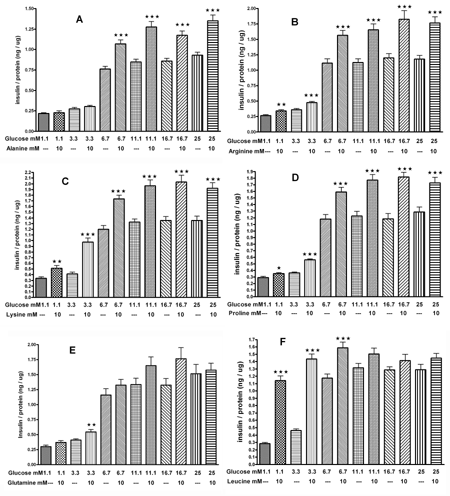 |
 |
Figure 5. The effect of glucose on the AA-mediated (10 mM) insulin secretion from INS-1E cells. A: L-alanine, B: L-arginine, C: L-lysine, D: L-proline, E: L-glutamine, F: L-leucine. Following a 30 min preincubation, we tested the effect of a 60-min incubation with 10 mM AAs and glucose on insulin secretion. Values are means ± SEM (n = 24). * p < 0.05, ** p < 0.01, *** p < 0.001 comparing the effect of glucose in the presence or absence of 10 mM AAs. |
|
Comparison of the effect of the equimolar concentration of 10 mM L-alanine, L-arginine, L-lysine, L-proline, L-leucine, and L-glutamine on glucose-stimulated insulin secretion from mouse islets and INS-1E cells
In the presence of 16.7 mM glucose, 10 mM of each AA, with the exception of L-glutamine, significantly enhanced glucose-stimulated insulin secretion from mouse islets (Figure 6A). Their potency on a molar basis ranks as follows: L-proline < L-alanine < L-lysine < L-leucine and L-arginine.
In the presence of 16.7 mM glucose, 10 mM of tested AAs with the exceptions of L-glutamine and L-leucine significantly enhanced glucose-induced insulin secretion from INS-1E cells (Figure 6B). Their potency on a molar basis ranks as follows: L-proline < L-alanine < L-lysine and L-arginine.
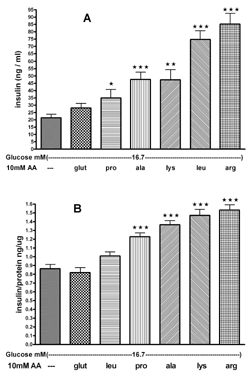 |
 |
Figure 6. The effect of 10 mM L-alanine, L-arginine, L-lysine, L-proline, L-glutamine and L-leucine on insulin secretion from mouse islets (A) and INS-1E cells (B) in the presence of 16.7 mM glucose. Following a 30 min preincubation, we tested the effect of a 60-min incubation with AAs on insulin secretion. Values are means ± SEM (n = 24). * p < 0.05, ** p < 0.01, *** p < 0.001 comparing the effect of glucose in the presence of different AAs versus glucose alone. |
|
Discussion
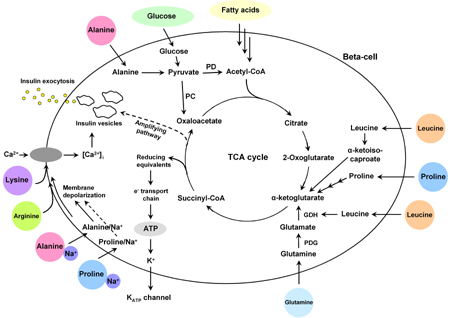
AA metabolism is essential to normal pancreatic beta-cell function [1, 7]. The mechanisms by which AAs confer their regulatory effects on beta-cells are complex, as illustrated in Figure 7, and may involve both transcriptional and mitochondrial effects [7]. The main focus of the present study was to clarify how the effects of AAs vary depending on concentration and prevailing glucose levels.
 |
 |
Figure 7. Schematic diagram of insulin secretion in beta-cells stimulated by glucose and amino acids. 1. Glucose enters the beta-cell by facilitated diffusion. Beta-cells have a high activity of both pyruvate dehydrogenase (PD) and pyruvate carboxylase (PC) to enhance the mitochondrial tricarboxylic acid (TCA) cycle activity and efficient ATP production. Enhancement of the TCA cycle activity results in closure of the ATP-sensitive K+ channel, depolarization of the plasma membrane, influx of Ca2+ and final fusion of insulin-containing granules with the plasma membrane. 2. AAs are accumulated within beta-cells by a number of different AA transport systems. AAs stimulate insulin secretion in beta-cells through three different mechanisms: (i) metabolism with generation of ATP (L-alanine, L-proline, L-leucine, L-glutamine); (ii) co-transport with sodium resulting in membrane depolarization (L-alanine, L-proline); (iii) uptake of cationic AAs (L-arginine, L-lysine) and membrane depolarization. AA: amino acid. PD: pyruvate dehydrogenase. PC: pyruvate carboxy-lase. PDG: phosphate-dependent glutaminase. GDH: glutamate dehydrogenase. TCA: tricarboxylic acid. |
|
McClenaghan et al. [12] studied the glucose-dependency of AAs on insulin secretion from the BRIN-BD11 pancreatic beta-cell line. They found that, at 1.1 mM glucose, 10 mM of L-alanine, L-arginine, L-glutamine, L-leucine, L-lysine and L-proline potentiated insulin secretion. In the presence of 16.7 mM glucose, the same AAs (10 mM), except for L-glutamine, potentiated glucose-induced insulin secretion [12]. In contrast, we did not find that 10 mM L-alanine enhanced insulin secretion from mouse islets or INS-1E cells at 1.1 or 3.3 mM glucose. A possible explanation for this discrepancy between our results and those of McClenaghan et al. [12] may be ascribed to differences in the clonal beta-cells.
In accordance with the study by McClenaghan et al. [12], a significant stimulation of insulin secretion was observed at 10 mM L-proline, L-lysine, L-arginine and L-leucine in the presence of 1.1 mM glucose. This indicates that even at low glucose, L-leucine and L-proline are able to enhance ATP production by entering into the tricarboxylic acid (TCA) cycle (Figure 7). L-arginine and L-lysine are transported into beta-cells, with direct depolarization of the plasma membrane at neutral pH, resulting in Ca2+ influx and activation of the exocytotic machinery (Figure 7) [5]. When glucose was increased from 1.1 to 25 mM, insulin release increased in both isolated mouse islets and INS-1E cells. With increasing glucose concentration, insulin secretion also progressively increased in response to L-proline, L-lysine, L-arginine and L-alanine, with the maximal effect obtained at 25 mM glucose.
As shown in Figure 7, the beta-cell has the following metabolic characteristics: 1. it expresses both GLUT-2 (high Km glucose transporter) and glucokinase; 2. the lactate dehydrogenase and plasma membrane mono-carboxylate pyruvate/lactate transporter activity is low, whereas the mitochondrial malate/aspartate shuttle activity is correspondingly high; and 3. it has a high activity of both pyruvate dehydrogenase and pyruvate carboxylase [7]. All these specific metabolic adaptations are geared to enhance mitochondrial TCA cycle activity, oxidative phosphorylation and efficient ATP production. An enhancement of the ATP/ADP ratio results in closure of the ATP-sensitive K+ channel, depolarization of the plasma membrane, opening of voltage-activated Ca2+ channels, influx of Ca2+ and finally, fusion of insulin-containing granules with the plasma membrane. Previous studies have shown that the addition of 10 mM L-alanine increased glucose consumption in BRIN-BD11 cells at 16.7 mM glucose [3]. Since co-transport of Na+ with L-alanine results in elevated beta-cell cytoplasmic Ca2+ which activates pyruvate dehydrogenase, then oxidation of pyruvate derived from glucose would be enhanced [13]. Vice versa, L-alanine was consumed at extremely high rates by both BRIN-BD11 cells and rat islets at higher glucose concentrations, since glucose promotes the uptake and utilization of L-alanine as a fuel through its metabolism [14].
At the level of 16.7 mM glucose, AAs increased the insulin secretion from mouse islets and INS-1E cells in a dose-dependent manner with the maximal effect being reached at 20 mM for L-proline, L-alanine, L-lysine and L-arginine. Whereas this was not the case for L-glutamine and L-leucine, the increase in insulin secretion from INS-1E cells was observed only at 20 mM L-leucine or L-glutamine. In accordance with this observation, L-leucine (10 mM) only increased insulin secretion from INS-1E cells at 1.1-6.7 mM glucose concentrations. At higher glucose concentrations, L-leucine-stimulated insulin secretion was inhibited.
L-leucine may stimulate insulin release through two different mechanisms [1, 7]. As shown in Figure 7, one mechanism involves transamination of L-leucine to α-ketoisocaproate and subsequent mitochondrial oxidation. The second promotes insulin release via allosteric activation of glutamate dehydrogenase (GDH) causing oxidation of glutamate to the Krebs cycle intermediate, alpha-ketoglutarate (α-KG). The involvement of GDH may explain the observation that, in contrast to other AAs, L-leucine stimulation of glutaminolysis and insulin secretion in perfused mouse islets was suppressed by high glucose [15], presumably due to increasing intracellular ATP and GTP, inhibiting GDH activity. Our results corroborate those of Li et al. [16], which described the glucose and leucine regulation of glutaminolysis and flux through GDH. Sener et al. [17] found that L-glutamine inhibited insulin secretion to 7.0-11.1 mM glucose in rat islets. In contrast, we found L-glutamine-stimulated insulin secretion in isolated mouse islets at 3.3-11.1 mM glucose (Figure 4E). Furthermore, it stimulated insulin secretion of INS-1E cells at only 3.3 mM glucose (Figure 5E).
At higher glucose concentrations, intracellular ATP and GTP production increased, thus inhibiting phosphate-dependent glutaminase (PDG) activity [15, 16]. Therefore, L-glutamine cannot enter into the TCA via glutamate and α-KG. The present study supports the previous finding that L-glutamine-induced insulin secretion disappears at high glucose [16]. Our study suggests that L-leucine stimulates glutaminolysis and insulin secretion.
Secretion from INS-1E cells may be dependent on the ratio of leucine/glucose concentrations. Only at 20 mM L-leucine or L-glutamine was glucose-induced (at 16.7 mM) insulin secretion potentiated by these two AAs. It should be noted that the effect of AAs on insulin secretion in vitro may differ significantly from insulin secretion in vivo due to neural, hormonal or metabolic regulation existing in vivo.
The sulphydryl amino acid, homocysteine, is an intermediate in the remethylation of methionine. It is transported into cells mainly via cysteine transporters [18]. Our study demonstrated an insulinostatic effect of DL-homocysteine on insulin release from isolated mouse islets and clonal beta-cells, which was only seen at the highest homocysteine concentration of 1000 μM together with normal (5.6 mM) and high (16.7 mM) glucose levels. The mechanism of insulin secretion inhibited by homocysteine remains unclear. However, the following explanations are possible [19]: 1. It may act on key regulatory steps of the TCA cycle in beta-cells, possibly via NAD depletion and reduction of glucose oxidation. It thus inhibits basal TCA cycle flux from other endogenous sources (glycogen, glutamate, fatty acids) and therefore reduces basal insulin secretion. 2. It inactivates adenylate cyclase leading to reduced levels of cAMP and inactivation of protein kinase A. and 3. Acute exposure to homocysteine results in disrupting the phospholipase C-protein kinase C pathways.
To date, no systematic data on the relative potency of the AAs tested in our study exists. It appeared that L-arginine is the most effective AA in stimulating insulin secretion of both mouse islets and INS-1E cells. The effect of L-leucine on insulin secretion differs between mouse islets and INS-1E cells. At higher glucose concentrations, L-leucine-mediated insulin secretion of INS-1E cells was inhibited, whereas it progressively stimulated insulin secretion by mouse islets with increasing glucose concentrations. The reason for this discrepancy is not known. One explanation could be that isolated islets contain both alpha- and beta-cells, while INS-1E cells are pure beta-cells. Another issue of potential importance is the difference in culture medium, i.e. pyruvate is present only in INS-1E cell but not in isolated islet medium.
AAs may play a critical role in maintaining the appropriate mass and function of islet beta-cells. This regulatory effect may be compromised by high circulating levels of glucose and/or fatty acids, which is observed in type 2 diabetes and may result in dysfunctional pancreatic beta- and alpha-cells [20-22]. In addition, circulating levels of AAs are changed acutely by diet and more chronically by disease states like type 2 diabetes, which may result in abnormal alpha- and beta-cell function. Interestingly, we found that physiological concentrations of L-alanine (0.1-1.0 mM), L-proline (0.1-1.0 mM) and L-lysine (0.1-1.0 mM) increased glucose-induced insulin secretion in vitro. Previous studies have shown that a combination of AAs in a mixed nutritional load is much more effective than an individual AA [1, 7]. We hypothesize that chronic exposure to abnormally high levels of certain AAs, present in the diabetic state, can alter target cell function and viability through specific changes in the expression of key regulatory genes and signal transduction elements, with serious consequences for islet cell function and viability. By identifying the transcription factors, genes and signal transduction proteins responsible for mediating the effects of AAs on cell function, new cellular targets involved in the control of islet cell function may be identified that would be relevant in the future development of novel treatment strategies for type 2 diabetes.
In conclusion, the present study demonstrates that L-arginine, L-lysine, L-alanine, L-proline, L-leucine and L-glutamine acutely stimulate insulin secretion from pancreatic beta-cells in a concentration- and glucose-dependent manner. Homocysteine is the only AA that was found to inhibit insulin secretion. In subsequent studies, we will explore the long-term impact of AAs on islet cell function, islet gene expression, and islet cell signal transduction. Further insight into the mechanism of AA-mediated beta-cell function and viability may help us to find new therapeutic strategies for diabetes.
Acknowledgments:
We would like to thank Dorthe Rasmussen, Kirsten Eriksen, Lene Trudsø, and Tove Skrumsager for skilled technical assistance. This study was supported by the Danish Diabetes Association, the faculty of Health Sciences, Aarhus University, Novo Nordisk Foundation and The Toyota Foundation.
References
- Newsholme P, Brennan L, Bender K. Amino acid metabolism, beta-cell function, and diabetes. Diabetes 2006. 55(Suppl 2):S39-S47. [DOD]
- Guidotti GG. Amino acid transport systems. Ital J Gastroenterol 1993. 25:37-41. [DOD]
- Brennan L, Shine A, Hewage C, Malthouse JG, Brindle KM, McClenaghan N, Flatt PR, Newsholme P. A nuclear magnetic resonance-based demonstration of substantial oxidative L-alanine metabolism and L-alanine-enhanced glucose metabolism in a clonal pancreatic beta-cell line. Diabetes 2002. 51:1714-1721. [DOD] [CrossRef]
- Yang J, Wong RK, Park M, Wu J, Cook JR, York DA, Deng S, Markmann J, Naji A, Wolf BA, Gao Z. Leucine regulation of glucokinase and ATP synthase sensitizes glucose-induced insulin secretion in pancreatic beta-cells. Diabetes 2006. 55:193-201. [DOD] [CrossRef]
- Smith PA, Sakura H, Coles B, Gummerson N, Proks P, Ashcroft FM. Electrogenic arginine transport mediates stimulus-secretion coupling in mouse pancreatic beta-cells. J Physiol 1997. 499:625-635. [DOD]
- McClenaghan NH, Barnett CR, Flatt PR. Na+ cotransport by metabolizable and nonmetabolizable amino acid stimulates a glucose-regulated insulin-secretary response. Biochem Biophys Res Commun 1998. 249(2):299-303. [DOD] [CrossRef]
- Newsholme P, Bender K, Kiely A, Brennan L. Amino acid metabolism, insulin secretion and diabetes. Biochem Soc Trans 2007. 35(5):1180-1186. [DOD] [CrossRef]
- Meigs JB, Jacques PF, Selhub J, Singer D, Nathan DM, Rifai N, D'Agostino RB, Wilson PW. Fasting plasma homocysteine levels in the insulin resistance syndrome. Diabetes Care 2001. 24:1403-1410. [DOD] [CrossRef]
- Jeppesen PB, Gregersen S, Poulsen CR, Hermansen K. Stevioside acts directly on the pancreatic beta-cell to secrete insulin: Actions independent of Camp and ATP-sensitive K-channel activity. Metabolism 2000. 49(2):208-214. [DOD] [CrossRef]
- Lacy PE, Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes 1967. 16:35-39. [DOD]
- Gregersen S, Hermansen K, Yanaihara N, Ahren B. Galanin fragments and analogues: effects on glucose-stimulated insulin secretion from isolated rat islets. Pancreas 1991. 6:216-220. [DOD]
- McClenaghan NH, Barnett CR, O'Harte FP, Flatt PR. Mechanisms of amino acid-induced insulin secretion from the glucose-responsive BRIN-BD11 pancreatic B-cell line. J Endocrinol 1996. 151(3):349-357. [DOD] [CrossRef]
- Civelek VN, Deeney JT, Shalosky NJ, Tornheim K, Hansford RG, Prentki M, Corkey BE. Regulation of pancreatic beta-cell mitochondrial metabolism: influence of Ca2+, substrate and ADP. Biochem J 1996. 318:615-621. [DOD]
- Dixon G, Nolan J, McClenaghan N, Flatt PR, Newsholme P. A comparative study of amino acid consumption by rat islet cells and the clonal beta-cell line BRIN-BD11 - the functional significance of L-alanine. J Endocrinol 2003. 179(3):447-454. [DOD] [CrossRef]
- Gao Zy, Li G, Najafi H, Wolf BA, Matschinsky FM. Glucose regulation of glutaminolysis and its role in insulin secretion. Diabetes 1999. 47:1535-1542. [DOD] [CrossRef]
- Li C, Najafi H, Daikhin Y, Nissim IB, Collins HW, Yudkoff M, Matschinsky FM, Stanley CA. Regulation of leucine-stimulated insulin secretion and glutamine metabolism in isolated rat islets. J Biol Chem 2003. 278(5):2853-3858. [DOD] [CrossRef]
- Sener A, Malaisse WJ. The stimulus-secretion coupling of amino acid-induced insulin release: insulinotropic action of branched-chain amino acids at physiological concentrations of glucose and glutamine. Eur J Clin Invest 1981. 11(6):455-460. [DOD] [CrossRef]
- Hultberg B. Extracellular concentration of homocysteine in human cell lines is influenced by specific inhibitors of cysteine transport. Clin Chem Lab Med 2004. 42(4):378-383. [DOD] [CrossRef]
- Patterson S, Flatt PR, Brennan L, Newsholme P, McClenaghan NH. Detrimental actions of metabolic syndrome risk factor, homocysteine, on pancreatic beta-cell glucose metabolism and insulin secretion. J Endocrinol 2006. 189:301-310. [DOD] [CrossRef]
- Dixon G, Nolan J, McClenaghan NH, Flatt PR, Newsholme P. Arachidonic acid, palmitic acid and glucose are important for the modulation of clonal pancreatic beta-cell insulin secretion, growth and functional integrity. Clin Sci (Lond) 2004. 106(2):191-199. [DOD]
- Hong J, Jeppesen PB, Nordentoft I, Hermansen K. Fatty acid-induced effect on glucagon secretion is mediated via fatty acid oxidation. Diabetes Metab Res Rev 2007. 23(3):202-210. [DOD] [CrossRef]
- Chen L, Jeppesen PB, Nordentoft I, Hermansen K. Stevioside counteracts beta-cell lipotoxicity without affecting acetyl CoA carboxylase. Rev Diabet Stud 2006. 3(4):178-188. [DOD] [CrossRef]
This article has been cited by other articles:
|