Review
| Rev Diabet Stud,
2010,
7(2):112-123 |
DOI 10.1900/RDS.2010.7.112 |
The Quest for Tissue Stem Cells in the Pancreas and Other Organs, and their Application in Beta-Cell Replacement
Isabelle Houbracken, Luc Bouwens
Cell Differentiation Lab, Diabetes Research Center, Vrije Universiteit Brussel (Free University of Brussels), Laarbeeklaan 103, 1090 - Brussels, Belgium
Address correspondence to: Luc Bouwens, e-mail: lucbo@vub.ac.be
Manuscript submitted July 4, 2010; resubmitted July 7, 2010; accepted July 10, 2010.
Keywords: stem cell, duct, progenitor, beta-cell, acinar, transdifferentiation, lineage tracing, reprogramming
Abstract
Adult stem cell research has drawn a lot of attention by many researchers, due to its medical hope of cell replacement or regenerative therapy for diabetes patients. Despite the many research efforts to date, there is no consensus on the existence of stem cells in adult pancreas. Genetic lineage tracing experiments have put into serious doubt whether β-cell neogenesis from stem/progenitor cells takes place postnatally. Different in vitro experiments have suggested centroacinar, ductal, acinar, stellate, or yet unidentified clonigenic cells as candidate β-cell progenitors. As in the rest of the adult stem cell field, sound and promising observations have been made. However, these observations still need to be replicated. As an alternative to committed stem/progenitor cells in the pancreas, transdifferentiation or lineage reprogramming of exocrine acinar and endocrine α-cells may be used to generate new β-cells. At present, it is unclear which approach is most medically promising. This article highlights the progress being made in knowledge about tissue stem cells, their existence and availability for therapy in diabetes. Particular attention is given to the assessment of methods to verify the existence of tissue stem cells.
Abbreviations: ATP - adenosine triphosphate; bHLH - basic helix-loop-helix; CA-II - carbonic anhydrase II; CD133 - cluster of differentiation 133 (surface glycoprotein, expressed by different stem and progenitor cells); c-Met - N-Methyl-N’-nitro-N-nitroso-guanidine HOS tranforming gene (encodes Met, also called hepatocyte growth factor receptor); c-Myc - cellular version of the myelocytomatosis oncogene (transcription factor regulating expression of many genes); Cre recombinase - type I topoisomerase (catalyzes site-specific recombination of DNA between loxP sites); CreERT - tamoxifen-induced Cre recombinase (fusion protein with the human estrogen receptor (ER) to induce recombinase activity); Cre/lox - recombination system to delete DNA sequences in living organisms (also termed Cre/loxP); DT - diphteria toxin; EGF - epidermal growth factor; ES cells - embryonic stem cells; Ex4- exendin-4; Glucagon-TetO system - glucagon tetracycline operator system; Hnf1b - hepatocyte nuclear factor 1 beta; Hnf1b-CreERT - mouse hepatocyte nuclear factor 1 beta (Hnf1b) promoter Cre-lox pulse-chase system; huCAII-CreERT - human carbonic anhydrase-II (CA-II) promoter Cre-lox pulse-chase system; iPS cells - induced pluripotent stem cells; Kras - Kirsten rat sarcoma (tissue signaling protein propagating growth factor); LIF - leukemia inhibitory factor; LoxP - locus of crossover in P1 (can be catalyzed by Cre); MafA - v-maf musculoaponeurotic fibrosarcoma oncogene homolog A (transcription factor necessary for beta-cell maturation); MAPK - mitogen-activated protein kinase; Ngn3 - neurogenin 3 (member of the bHLH family of transcription factors expressed in the nervous system); Pax4 - paired box gene 4 (transcription factor involved in fetal and pancreas development); PDL - partial duct ligation; Pdx1 - pancreatic and duodenal homeobox 1 (transcription factor necessary for pancreas development); siRNA - short interfering ribonucleic acid; STAT3 - signal transducer and activator of transcription 3; TGF-α - transforming growth factor α
Introduction
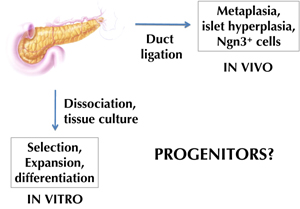
Islet cell transplantation with the Edmonton and related protocols has provided proof-of-principle for cell replacement therapy as a valid approach for the treatment of type 1 diabetes [1-5]. One limitation in its clinical application is the shortage of donor organs, from which islet cells can be isolated. Therefore, alternative sources are needed for transplantable β-cells, or β-like cells. Pluripotent stem cells like embryonic stem (ES) cells, or induced pluripotent stem (iPS) cells, would be attractive sources, given their unlimited capacity for expansion. However, it is assumed that these undifferentiated cells need to go through numerous successive stages of differentiation, as during embryonic development, to become appropriately differentiated cells like β-cells. This makes the differentiation protocol very complex. But one could hope to shortcut this by using cells already committed to the pancreatic fate. Such cells could be adult pancreatic stem cells. These cells could provide targets for pharmacological intervention aiming at β-cell regeneration in diabetes patients. However, despite a wealth of studies in the last decade, it is still uncertain whether stem cells, or endocrine progenitor cells, actually reside in the adult pancreas (Figure 1). We distinguish between histological studies aimed at identifying progenitor cells inside pancreatic tissue, and in vitro studies performed on dissociated pancreatic tissue.
 |
 |
Figure 1. Approaches to identify progenitor cells in the adult pancreas. Two major approaches have been applied to find evidence for adult β-cell neogenesis in the adult pancreas (i.e. formation of new β-cells from progenitor cells). One approach consisted of isolating progenitor cells, and stimulating their neogenic activity in vitro. This gave rise to c-Met- [32] or aldehyde dehydrogenase [33] expressing cells, for example. The other approach was based on causing tissue injury to stimulate progenitor cells in vivo, for example, cells that express Ngn3 following duct ligation [25]. In both cases, genetic lineage tracing still needs to be done to identify the stem/progenitor cell source. |
|
Are there progenitor cells in adult pancreatic tissue?
Interest in adult islet cell progenitors, or precursors, predates the stem cell concept. Even in the earliest histological studies on pancreatic islets, a relationship was proposed between exocrine and endocrine tissue. Some proponents of this relationship suggested acinar cells, and others ductal cells, as possible islet precursors [6-12]. In the wake of the stem cell "hype" that occurred around the turn of the last century, "dormant" or "facultative" stem cells were thought to reside in the adult gland, possibly in the exocrine ducts [13]. Alternatively, it was proposed that fully differentiated exocrine acinar, and/or duct cells, were able to transdifferentiate into endocrine islet cells [14]. Histological evidence, mostly indirect or correlative, was used to support these hypotheses for β-cell neogenesis. The problem remains that histological studies only give snapshot images, from which it is very difficult to reconstruct the entire picture.
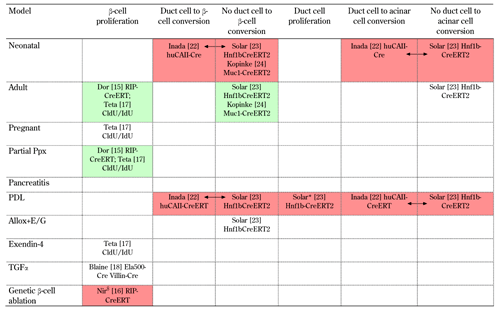
In 2004, Dor et al. used the innovative approach of genetic pulse-chase labeling to test the neogenesis hypothesis in adult mice [15]. Their approach was based on an insulin promotor-driven Cre-lox labeling system that is dependent on a tamoxifen-pulse for specific labeling of β-cells at a chosen time point (insulin-CreERT). After that time point, β-cells and their progeny remained stably labeled and could be chased. The authors observed, that within a time frame of one year post-labeling, the proportion of labeled β-cells remained constant. Whereas, it should have decreased if new β-cells had originated from stem cells, or other cell types. In fact, putative progenitor cells are not expected to transcribe insulin at the time of labeling. Therefore, they should provide unlabeled progeny that could "dilute" the labeled islet cells. In the same study, partial pancreatectomy was used as a stimulus for partial regeneration. However, there was no dilution of labeled β-cells in the regenerating tissue. This observation made neogenesis from progenitor cells unlikely (Table 1).
Table
1.
Overview of lineage tracing evidence for cellular conversion in the pancreas (beta-cell and duct cell proliferation and conversion) |
|
 |
 |
Legend:
Several studies with lineage tracing evidence for cellular conversions in the pancreas have been performed. Different models have been used: in vivo, and in vitro, from normal development to genetic alterations. Most studies are mutually confirmatory, but some are controversial. Whilst the study of Inada et al. proposed adult duct cells as multipotent progenitors [22], the studies by Solar et al. and Kopinke et al. found no evidence for a ductal derivation of other adult pancreatic cell types [23, 24]. There are no published studies based on lineage tracing of adult stem or progenitor cell markers. Indicated are: the first author, the reference number, and the (promoter) construct used for lineage tracing. Studies shown in green on the same row confirm each other. Studies shown in red, are conflicting (double arrows indicate studies with opposing results). * In PDL, the studies of Solar et al. [23] and Desai et al. [30] seem to conflict on the contribution of acinar cells to the ductal complexes. However, in Desai et al. [30], no quantification was performed. $ Nir et al. [16] and Thorel et al. [67] appear to be contradictory. However, they used different genetic β-cell ablation models (70-80% ablation in insulin-rtTA TET-DTA, and more than 99% in RIP-DTR mice, respectively). Neonatal: analysis during neonatal period with labeling around birth and analysis at least 2 weeks later. Adult: analysis during adult life. Pregnant: analysis during pregnancy. Partial Ppx: partial pancreatectomy (50-80%). Pancreatitis: acute and chronic pancreatitis induced by caerulein injections. PDL: partial duct ligation. Allox+E/G: β-cell-specific ablation by alloxan followed by EGF/gastrin treatment. Exendin-4: exendin-4 injections. TGF-α: TGF-α overexpression. Genetic β-cell ablation: β-cell-specific ablation in insulin-rtTA TET-DTA or RIP-DTR mice. Pax4: Pax4 overexpression. Ngn3, Pdx1, MafA: overexpression of Ngn3, Pdx1, and MafA by adenoviruses. Kras mutation: mutated Kras (oncogene) overexpression. In vitro: culture of rat or mouse exocrine cells. |
|
Similar findings were obtained in mice, in which 70-80% of the β-cells had been genetically ablated [16] (Table 1). Another innovative non-genetic lineage tracing technique, based on serial thymidine analog labeling, confirmed these findings in normal adult, pregnant, 50% partial pancreatectomized, and exendin-4 (Ex4) treated mice [17] (Table 1). In a recent study, Blaine et al. showed that insulin-positive cells adjacent to hyperplastic ductal epithelium arose from pre-existing insulin-positive cells, after transforming growth factor α (TGF-α) stimulation in vivo [18] (Table 1). Previously, it had been suggested that these endocrine cells arose from the 'multipotent' metaplastic ductal epithelium [19, 20]. These experiments demonstrated that in postnatal life, β-cells do not derive from stem cells, but rather renew and expand by the proliferation of β-cells already present shortly after birth.
The experiments discussed above do not rule out the possibility that under specific conditions "dormant" progenitor cells might become activated to generate new β-cells, i.e. performing β-cell "neogenesis". It was considered that generation of new β-cells occurred in the partial duct ligation (PDL) model. PDL is an experimental model, in which tissue injury is caused to part of the pancreas. In this model, β-cell number reduplicated within a week post-ligation in the ligated portion of the pancreas [21].
Inada et al. used another genetic lineage tracing model, with human carbonic anhydrase-II (CA-II) promoter acting as a driver of the Cre-lox pulse-chase system (huCAII-CreERT) [22]. This was intended to allow tracing of duct cells which express CA-II, and was applied to duct-ligated mice. The authors found that a significant proportion of β-cells expressed the label in the duct-ligated pancreas. Also, labeled β-cells and acinar cells were found in normal neonates a few weeks after tamoxifen pulse (Table 1). However, more recently, Solar et al. found completely opposite results, using a genetic lineage tracing system for duct cells. Their system was based on the mouse hepatocyte nuclear factor 1 beta (Hnf1b) promoter as driver (Hnf1b-CreERT) [23] (Table 1). Hnf1b is a transcription factor that is expressed specifically by all duct cells in embryonic and postnatal pancreas. The authors found that early embryonic duct cells were multipotent progenitors for all pancreatic lineages, but their differentiation potency became gradually restricted. At the end of gestation and after birth, duct cells were only capable of generating other duct cells, and they did not contribute further to β-cell mass, or to other lineages. According to this study, duct cells could not generate β-cells in normal neonatal and adult life, after pancreatic duct ligation, and in a model of β-cell regeneration following alloxan-destruction.
At present, the discrepancy between the Inada and the Solar finding is difficult to explain. A drawback of the Inada study is that it used a human fragment of the CAII promoter to direct Cre-recombinase expression in mice. This could have caused misexpression in cell types other than duct cells. Solar et al. ascertained that expression of Cre-recombinase enzyme and reporter enzyme beta-galactosidase was restricted to duct cells. Furthermore, they demonstrated by immunohistochemistry that all duct cells expressed Hnf1b and Cre-enzyme. This ruled out the existence of a postnatal duct cell subset that might have been overlooked by the tracing system that did not ascertain 100% beta-galactosidase labeling of duct cells due to inefficient recombination. More recently, another study appeared, using Mucin-1 genetic labeling to trace exocrine acinar and duct cells that express this gene [24]. This study failed to provide evidence for a contribution by these cells to the islet cell compartment, in normal neonatal and adult life; thereby confirming the conclusion of the Solar study (Table 1).
In another study of duct-ligated pancreas, Xu et al. reported the appearance of neurogenin 3 (Ngn3)-positive cells in the duct-ligated pancreas (Figure 1) [25]. These cells were shown to be endocrine progenitors. This conclusion was based on the observation that the cells were able to differentiate into different endocrine cell types ex vivo, when isolated and transferred into embryonic pancreatic explants as a suitable microenvironment. Ngn3 expression was required for the observed β-cell mass expansion after duct ligation, since Ngn3-silencing with short interfering (si)RNA partially inhibited the β-cell numerical increase. However, it is not clear where these Ngn3+ cells originated. They were frequently observed in the vicinity of ducts, but this could be misleading, as ducts and islets are the only epithelial structures remaining after duct ligation due to acinoductal metaplasia.
The recent demonstration of Ngn3 expression in islet β-cells [26] might challenge the earlier conclusion that Ngn3+ cells are true progenitors. It remains to be ruled out that pre-existing β-cells might upregulate Ngn3 (and proliferate) in the partial duct ligation model. In insulin-Cre tracer mice, labeled Ngn3-positive cells were not detected by immunohistochemical staining. This result suggested that the cells were not derived from β-cells. However, Ngn3 detection by immunohistochemistry is difficult [27]. A better way to examine the possibility that β-cells can give rise to Ngn3-positive cells would be to cross insulin-Cre tracer mice with Ngn3-reporter mice. Also, adult acinar cells can be stimulated to express Ngn3 [28, 29] (Figure 2). This finding suggests that acinar cells can be a source of Ngn3+ precursors in the PDL model. On the other hand, Desai et al. found no evidence for a contribution of acinar cells to β-cells after PDL in elastase-tracing mice [30] (Table 2).
 |
 |
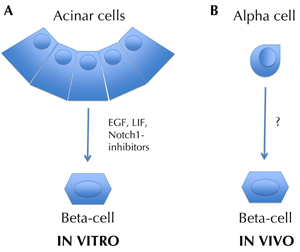
Figure 2. Transdifferentiation in the pancreas. Transdifferentiation, or reprogramming, is the conversion of one differentiated cell type into another. For example, in the presence of LIF and EGF, acinar cells have been shown to transdifferentiate into mature β-cells in vitro. This was significantly increased by inhibiting Notch1-Hes1 signaling [28, 53]. α-cells have been shown to transdifferentiate into β-cells in vivo, after genetic ablation of the majority of β-cells [67]. In the latter case, it is not known which factors stimulated this phenomenon. Non-genetic and genetic lineage tracing methods, respectively, have been used in these studies to identify the origin cells. |
|
Table
2.
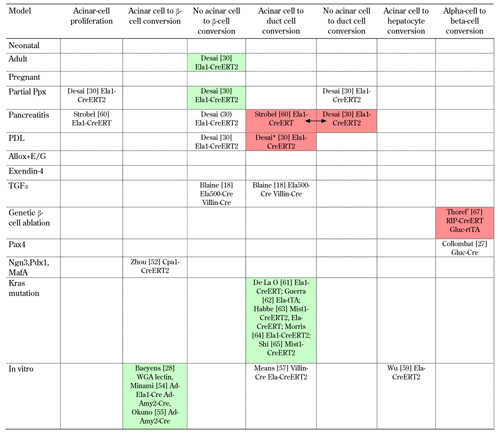
Overview of lineage tracing evidence for cellular conversion in the pancreas (acinar cell proliferation and conversion) |
|
 |
 |
Legend:
Indicated are: the first author, the reference number, and the (promoter) construct used for lineage tracing. Studies shown in green on the same row confirm each other. Studies shown in red, are conflicting (double arrows indicate studies with opposing results). * In PDL, the studies of Solar et al. [23] and Desai et al. [30] seem to conflict on the contribution of acinar cells to the ductal complexes. However, in Desai et al. [30], no quantification was performed. $ Nir et al. [16] and Thorel et al. [67] appear to be contradictory. However, they used different genetic β-cell ablation models (70-80% ablation in insulin-rtTA TET-DTA, and more than 99% in RIP-DTR mice, respectively). See also legend of Table 1. |
|
In summary, at present there is no clear evidence for the hypothesis that new islet cells originate postnatally from ducts. Insulin promoter-driven cell tracing has not yet been used to demonstrate the existence of β-cell neogenesis postnatally after duct ligation or in other conditions. Also, there is no conclusive histological evidence for the existence of pancreatic or endocrine stem/progenitor cells in the adult organ.
Can the existence of pancreatic/endocrine progenitor cells be demonstrated ex vivo?
It is possible that adult pancreatic tissue harbors a not yet identified population of stem/progenitor cells. Such "dormant" progenitors could represent an additional source of cells that may be capable of activation in artificial culture conditions, e.g. in the presence of necessary growth factors, feeder cells, or extracellular matrix components. Therefore, researchers have tried several culture conditions on dissociated pancreatic tissue to find out if any of the pancreatic cells are able to differentiate into β-cells. This procedure is called the "gardening approach" (Figure 1). Sometimes, this approach was based on protocols that work with other tissue stem cell types, and at other times, different combinations of growth factors, hormones, or cytokines were tested randomly.
Clonally proliferating cells have been found that showed some differentiation plasticity in vitro, evidenced by exhibiting neural and endocrine cell markers [31]. In another study, the c-Met receptor for hepatocyte growth factor was used prospectively to sort cells that could form clonal colonies expressing multiple pancreatic and non-pancreatic markers in vitro. The latter included liver, stomach, and intestine markers [32]. Sorted cell preparations expressing aldehyde dehydrogenase enzymatic activity, and that were enriched in centroacinar and ductal cells, were able to form self-renewing "pancreatospheres" in suspension culture. In these organoids, both endocrine and exocrine differentiation occurred [33]. Also, in the study of Ramiya et al., digested pancreatic ductal tissue (from prediabetic non-obese diabetic mice) could be subcultured over a long period [34]. This tissue could be induced to produce functioning islets containing α, β, and δ cells. Unfortunately, no lineage tracing was performed to unequivocally identify the origin cells.
CD133 expression is a marker frequently associated with stem cells. Based on this marker, cells were obtained from adult mouse pancreas. The cells showed a high proliferative capacity, but were committed to the ductal lineage [35]. Cells with similar characteristics, but isolated perinatally, showed a certain degree of differentiation plasticity. This observation is in accordance with the finding that duct cells gradually lose their differentiation plasticity during embryonic-fetal life, and become restricted to the ductal lineage postnatally [23]. Also, the CD133 marker has been shown to be expressed on all differentiated centroacinar and duct cells in adult pancreas [36]. This means that it cannot be used for prospective isolation of putative pancreatic stem cells.
Mato et al. purified a population of pancreatic stellate cells from lactating rats [37]. These cells expressed the ATP-binding cassette transporter frequently associated with stem cells, and could be grown for over 2 years as a fibroblast-like monolayer. When plated on extracellular matrix and given a cocktail of growth factors, the cells expressed phenotypic markers characteristic of β-cells. In another study, nestin-positive cells from pancreatic islets and ducts showed extended proliferative capacity, and appeared to be multipotent [38]. These cells showed both liver and exocrine pancreas markers, and displayed a ductal/endocrine phenotype after differentiation.
It is largely irrelevant whether the abovementioned observations are material to pathophysiological conditions, or represent in vitro artefacts, provided that this "bioengineering" approach can lead us to the derivation of transplantable β-like cells. However, such studies often lead to β-like cells, in which the expression of β-cell marker genes and proteins occurs at very low levels compared to genuine islet β-cells. More studies are needed demonstrating that the obtained insulin-expresssing β-like cells are capable of regulating blood glycemia in vivo.
It is also still questionable whether progenitor cells reside within the human pancreas. In a study with duct tissue from human pancreas, using defined culture medium and extracellular matrix, insulin-producing cells originated from the epithelium [39]. When the same conditions were applied to human preparations, from which the contaminating β-cells had first been removed, no new β-cells could be formed [40]. The reason for this discrepancy is unclear, but it emphasizes again the need to use lineage tracing in future experiments of this kind. This also applies to the study by Suarez-Pinzon et al. [41]. The authors reported an increase in the number of β-cells after treatment of a mixture of human islet, duct, and acinar cells with epidermal growth factor and gastrin. It is unclear whether the resulting β-cell are a consequence of neogenesis from duct cells.
Some studies have followed another approach to demonstrate the presence of islet progenitor cells in adult pancreatic epithelium, namely by co-transplantation of adult pancreatic and fetal tissue. The rationale for this approach was that the combination of fetal cells, and the in vivo environment of the graft, might provide the necessary factors to promote differentiation of putative endocrine progenitors present in the adult pancreatic tissue. It is now twenty years since it was first reported that co-transplantation of rat non-endocrine pancreatic tissue with fetal tissue seemed to induce islet formation in the graft [42]. More recently, this was successfully demonstrated with human cells [43]. In the recent study, islet cells in the graft were from donor tissue, as evidenced by genetic labeling. Interestingly, in the previous studies, preparations may have still contained some contaminating β-cells at study start. Co-transplantation of affinity-purified human duct cells with stromal feeder cells was found to cause the appearance of β-cells in the graft [44]. These studies suggest that there may be cells endowed with a certain differentiation plasticity even in the adult human pancreas. Such cells might be harnessed to generate β-cells in defined culture conditions, although these conditions still remain a "black box" at present. The cellular progenitor characteristics are still unknown. The same applies for the question, whether they represent true self-renewing stem cells, or mature cells that are still endowed with a specific plasticity (see next section).
Transdifferentiation
Transdifferentiation is the conversion of one differentiated cell type into another (Figure 2). Although this approach has already been known for many years [45-47], it has become more popular recently under the term "cellular reprogramming". β-cell neogenesis may result from the differentiation of putative stem/progenitor cells, i.e. cells that have not yet reached a "terminally differentiated state". Alternatively, it could result from the transdifferentiation of mature pancreatic cell types.
Amongst other examples, it was found that introducing genes for three, or four, transcription factors, could convert somatic cells, like skin fibroblasts, into pluripotent stem cells [48-50], or into mature neurons, for example [51], depending on the nature of the transcription factors used. Similarly, in vivo delivery of two, or three, transcription factor-encoding genes in mouse pancreas, e.g. Ngn3, Pdx1, and MafA, led to the transdifferentiation of acinar cells into functional β-cells [52] (Table 2). Even more exciting is the possibility of inducing transdifferentiation with growth factors, or cytokines, that do not require viral vector or gene insertion. The in vitro conversion of normal rat exocrine acinar cells into functional β-cells was reported first by Baeyens et al. [53] (Figure 2). This group used a combination of two factors in the culture medium, namely epidermal growth factor (EGF) and leukemia inhibitory factor (LIF). After only 3 days of culture in this medium, approximately 10% of epithelial cells expressed insulin and other β-cell markers.
Other researchers have reported on in vitro transdifferentiation of mouse acinar cells into β-like cells, which was induced by EGF and nicotinamide. This was achieved with cells from normal mice, and from diabetic mice [54, 55] (Table 2). However, this approach was associated with lower efficiency. When EGF and LIF was supplemented, and Notch signaling was inhibited, up to two-thirds of rat exocrine cells acquired the β-cell phenotype [28]. In this study, non-genetic lineage tracing was performed with a lectin that specifically labeled acinar cells, and which demonstrated the acinar origin of the newly formed β-cells (Table 2). The reprogrammed acinar cells were able to normalize blood glucose levels after transplantation into diabetic animals, thus demonstrating their functional maturity. Although the cells initially showed some phenotypic differences with normal islet β-cells, indicating relative immaturity; they became undistinguishable from normal β-cells one week after transplantation.
The reprogramming of acinar cells to β-cells requires the two factors, EGF and LIF. It also depends on activation of STAT3 and mitogen-activated protein kinase (MAPK) pathways, and on the expression of Ngn3 during an intermediate phase of the transdifferentiation process [29]. As reported earlier with acinoductal transdifferentiation (i.e. reprogramming of acinar cells into duct cells [56, 57] (Table 2)), the acinar cells seem to go through an intermediate phase of relative dedifferentiation. Thereby they acquire characteristics of progenitor cells. They can also transdifferentiate into hepatocyte-like cells [58, 59] (Table 2), which indicates the multipotency of acinar pancreatic cells, and their ability to become reprogrammed in different microenvironments. However, it remains unclear whether acinar cells can also exhibit this potential in vivo under pathophysiological conditions (without gene transduction). Genetic lineage tracing, allowing specific acinar cell labeling (elastase-CreERT), revealed that conversion of acinar cells into endocrine cells did not occur. Although acinoductal transdifferentiation was demonstrated by this acinar-specific tracing technique. This was evident in different experimental conditions such as acute and chronic pancreatitis, partial duct ligation, and TGF-α stimulation [18, 30, 60] (Table 2). Also, acinoductal conversion was demonstrated when mutated Kras was expressed in acinar cells [61-65] (Table 2). Bonal et al. suggested that inactivation of c-Myc in pancreatic cells, leads to the transdifferentiation of acinar cells into adipocytes [66]. However, their tracing was based on the pancreas progenitor-specific pancreatic and duodenal homeobox 1 (Pdx1)-Cre, and not on an acinar-specific tracing technique. All these studies indicate a high level of plasticity for acinar cells in vivo, and in vitro. Therefore, these cells could serve as a progenitor pool for several cell types. Analogous to the in vitro transdifferentiation of acinar cells to β-cells [53], it would be interesting to study the effect of factors like EGF, and LIF, on acinar cells in vivo. Also, the transdifferentiation capacity of human exocrine cells needs to be demonstrated, before this knowledge can be translated to cell replacement therapy.
Another interesting example of transdifferentiation was recently reported by Herrera's group, namely α-cells converting in vivo into β-cells [67] (Figure 2). This study made use of efficient genetic ablation of β-cells, using the diphteria toxin (DT) receptor under the insulin promoter. After DT administration, more than 99% of the β-cells were ablated. In mice that received exogenous insulin for survival, there was a slow and partial regeneration of β-cells. Genetic lineage tracing (glucagon-TetO system) revealed that α-cells contributed to this β-cell regeneration (Table 2). Also, α-cell to β-cell transdifferentiation was shown to occur in mice which overexpress Pax4 in mature α-cells [27]; and in a PDL plus alloxan model, where all β-cells that appeared after treatment were neogenic [68, 69] (Table 2).
In some studies, transdifferentiation of mature cells (like acinar or α-cells) with, or without, a prior dedifferentiation event, could explain observations attributed to stem cell differentiation. Now, it is clear that future studies examining β-cell neogenesis, and putative tissue stem cells, will require rigorous testing by genetic or other lineage tracing.
Non-pancreatic stem/progenitor cells
Some adult tissues contain stem/progenitor cells. These cells are responsible for normal tissue renewal. Usually, their differentiation potential is considered to be lineage-restricted i.e. confined to cells of the host tissue/organ. However, recent work has demonstrated that they can be transdifferentiated or transdetermined to other types of tissue. Transdetermination means switching of lineage commitment in lineage-determined, but not terminally differentiated, cells. This approach offers the opportunity for non-pancreatic tissues to generate insulin-expressing cells.
Multiple tissues have been shown to differentiate into insulin-expressing cells, including liver [70-75], bone marrow [76-82], intestine [83], neural tissue [84], epidermis [85], salivary gland [86], and fibroblasts [87]. These studies have been reviewed in this issue and elsewhere [88-90]. However, data on the plasticity of adult stem cells remain controversial. Some of the transdifferentiation and transdetermination events have been attributed to cell fusion. Others are claimed to have only a supporting role in the endogenous regeneration capacity of the pancreas. Also, lineage tracing has not always been performed in these studies. Finally, many of these studies needed genetic manipulations, which are difficult to apply safely to the clinic.
Conclusions
Similar debates, concerning stem cells in other organs, highlight a major problem in the adult stem cell field. Namely, many research teams are not able to reproduce the seemingly promising results of others [91]. This may be the reason why we cannot yet make a clear conclusion on the existence, and identity, of adult stem cells in the pancreas. In vivo genetic lineage tracing experiments have indicated that stem cells do not participate to the β-cell mass postnatally.
However, in vitro experiments have provided promising observations on what might represent stem cells. There is a real need to explore further the relationships between the expression of stem cell markers like c-Met, CD133 and aldehyde dehydrogenase, the expression of Ngn3, clonigenicity, and/or self-renewal. Regarding the pancreas, we still await the formation of organoids containing the different mature cell types, from a single adult stem cell in culture, similar to the work already done with gut stem cells [92].
Transdifferentiation of other pancreatic cell types, like acinar cells and α-cell to β-cell, represents an alternative to stem cells. It may find its way to clinical application, if the results obtained in rodents can be translated to human cells.
Disclosures (conflict of interests statement): The authors report no conflict of interests.
Acknowledgments:
I.H. is a research fellow of the Fund for Scientific Research-Flanders (FWO-Vlaanderen).
References
- Keymeulen B, Ling Z, Gorus FK, Delvaux G, Bouwens L, Grupping A, Hendrieckx C, Pipeleers-Marichal M, Van Schravendijk C, Salmela K, et al. Implantation of standardized beta-cell grafts in a liver segment of IDDM patients: graft and recipients characteristics in two cases of insulin-independence under maintenance immunosuppression for prior kidney graft. Diabetologia 1998. 41(4):452-459. [DOD] [CrossRef]
- Ryan EA, Lakey JR, Rajotte RV, Korbutt GS, Kin T, Imes S, Rabinovitch A, Elliott JF, Bigam D, Kneteman NM, et al. Clinical outcomes and insulin secretion after islet transplantation with the Edmonton protocol. Diabetes 2001. 50(4):710-719. [DOD] [CrossRef]
- Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med 2000. 343(4):230-238. [DOD] [CrossRef]
- Street CN, Lakey JR, Shapiro AM, Imes S, Rajotte RV, Ryan EA, Lyon JG, Kin T, Avila J, Tsujimura T, et al. Islet graft assessment in the Edmonton Protocol: implications for predicting long-term clinical outcome. Diabetes 2004. 53(12):3107-3114. [DOD] [CrossRef]
- Warnock GL, Kneteman NM, Ryan EA, Rabinovitch A, Rajotte RV. Long-term follow-up after transplantation of insulin-producing pancreatic islets into patients with type 1 (insulin-dependent) diabetes mellitus. Diabetologia 1992. 35(1):89-95. [DOD] [CrossRef]
- Granger A, Kushner JA. Cellular origins of beta-cell regeneration: a legacy view of historical controversies. J Intern Med 2009. 266(4):325-338. [DOD] [CrossRef]
- Laguesse E. Sur l'evolution des ilots endocrines dans le pancreas de l'homme adulte. Arch d'anat micr 1908-1910. 11:1-93. [DOD]
- Bensley RR. Studies on the pancreas of the guinea pig. Am J Anat 1911. 12:297-388. [DOD] [CrossRef]
- Laguesse E. Le pancreas. Revue generale d'histologie 1906. [DOD]
- Leduc EH, Jones EE. Acinar-islet cell transformation in mouse pancreas. J Ultrastruct Res 1968. 24(1):165-169. [DOD] [CrossRef]
- Setalo G. Electron microscopic investigation of acino-insular transformation in the rat. Acta Biol Acad Sci Hung 1967. 18(3):323-333. [DOD]
- Setalo G. Light microscopic demonstration of acino-insular transformation. Acta Morphol Acad Sci Hung 1970. 18(4):359-367. [DOD]
- Bonner-Weir S. Perspective: Postnatal pancreatic beta cell growth. Endocrinology 2000. 141(6):1926-1929. [DOD] [CrossRef]
- Bouwens L. Transdifferentiation versus stem cell hypothesis for the regeneration of islet beta-cells in the pancreas. Microsc Res Tech 1998. 43(4):332-336. [DOD] [CrossRef]
- Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature 2004. 429(6987):41-46. [DOD] [CrossRef]
- Nir T, Melton DA, Dor Y. Recovery from diabetes in mice by beta cell regeneration. J Clin Invest 2007. 117(9):2553-2561. [DOD] [CrossRef]
- Teta M, Rankin MM, Long SY, Stein GM, Kushner JA. Growth and regeneration of adult beta cells does not involve specialized progenitors. Dev Cell 2007. 12(5):817-826. [DOD] [CrossRef]
- Blaine SA, Ray KC, Anunobi R, Gannon MA, Washington MK, Means AL. Adult pancreatic acinar cells give rise to ducts but not endocrine cells in response to growth factor signaling. Development 2010. 137(14):2289-2296. [DOD] [CrossRef]
- Song SY, Gannon M, Washington MK, Scoggins CR, Meszoely IM, Goldenring JR, Marino CR, Sandgren EP, Coffey RJ Jr, Wright CV, et al. Expansion of Pdx1-expressing pancreatic epithelium and islet neogenesis in transgenic mice overexpressing transforming growth factor alpha. Gastroenterology 1999. 117(6):1416-1426. [DOD] [CrossRef]
- Wang TC, Bonner-Weir S, Oates PS, Chulak M, Simon B, Merlino GT, Schmidt EV, Brand SJ. Pancreatic gastrin stimulates islet differentiation of transforming growth factor alpha-induced ductular precursor cells. J Clin Invest 1993. 92(3):1349-1356. [DOD] [CrossRef]
- Wang RN, Kloppel G, Bouwens L. Duct- to islet-cell differentiation and islet growth in the pancreas of duct-ligated adult rats. Diabetologia 1995. 38(12):1405-1411. [DOD] [CrossRef]
- Inada A, Nienaber C, Katsuta H, Fujitani Y, Levine J, Morita R, Sharma A, Bonner-Weir S. Carbonic anhydrase II-positive pancreatic cells are progenitors for both endocrine and exocrine pancreas after birth. Proc Natl Acad Sci U S A 2008. 105(50):19915-19919. [DOD] [CrossRef]
- Solar M, Cardalda C, Houbracken I, Martin M, Maestro MA, De Medts N, Xu X, Grau V, Heimberg H, Bouwens L, et al. Pancreatic exocrine duct cells give rise to insulin-producing beta cells during embryogenesis but not after birth. Dev Cell 2009. 17(6):849-860. [DOD] [CrossRef]
- Kopinke D, Murtaugh LC. Exocrine-to-endocrine differentiation is detectable only prior to birth in the uninjured mouse pancreas. BMC Dev Biol 2010. 10:38. [DOD] [CrossRef]
- Xu X, D'Hoker J, Stange G, Bonne S, De Leu N, Xiao X, Van de Casteele M, Mellitzer G, Ling Z, Pipeleers D, et al. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell 2008. 132(2):197-207. [DOD] [CrossRef]
- Wang S, Jensen JN, Seymour PA, Hsu W, Dor Y, Sander M, Magnuson MA, Serup P, Gu G. Sustained Neurog3 expression in hormone-expressing islet cells is required for endocrine maturation and function. Proc Natl Acad Sci U S A 2009. 106(24):9715-9720. [DOD] [CrossRef]
- Collombat P, Xu X, Ravassard P, Sosa-Pineda B, Dussaud S, Billestrup N, Madsen OD, Serup P, Heimberg H, Mansouri A. The ectopic expression of Pax4 in the mouse pancreas converts progenitor cells into alpha and subsequently beta cells. Cell 2009. 138(3):449-462. [DOD] [CrossRef]
- Baeyens L, Bonne S, Bos T, Rooman I, Peleman C, Lahoutte T, German M, Heimberg H, Bouwens L. Notch signaling as gatekeeper of rat acinar-to-beta-cell conversion in vitro. Gastroenterology 2009. 136(5):1750-1760. [DOD] [CrossRef]
- Baeyens L, Bonne S, German MS, Ravassard P, Heimberg H, Bouwens L. Ngn3 expression during postnatal in vitro beta cell neogenesis induced by the JAK/STAT pathway. Cell Death Differ 2006. 13(11):1892-1899. [DOD] [CrossRef]
- Desai BM, Oliver-Krasinski J, De Leon DD, Farzad C, Hong N, Leach SD, Stoffers DA. Preexisting pancreatic acinar cells contribute to acinar cell, but not islet beta cell, regeneration. J Clin Invest 2007. 117(4):971-977. [DOD] [CrossRef]
- Seaberg RM, Smukler SR, Kieffer TJ, Enikolopov G, Asghar Z, Wheeler MB, Korbutt G, van der Kooy D. Clonal identification of multipotent precursors from adult mouse pancreas that generate neural and pancreatic lineages. Nat Biotechnol 2004. 22(9):1115-1124. [DOD] [CrossRef]
- Suzuki A, Nakauchi H, Taniguchi H. Prospective isolation of multipotent pancreatic progenitors using flow-cytometric cell sorting. Diabetes 2004. 53(8):2143-2152. [DOD] [CrossRef]
- Rovira M, Scott SG, Liss AS, Jensen J, Thayer SP, Leach SD. Isolation and characterization of centroacinar/terminal ductal progenitor cells in adult mouse pancreas. Proc Natl Acad Sci U S A 2010. 107(1):75-80. [DOD] [CrossRef]
- Ramiya VK, Maraist M, Arfors KE, Schatz DA, Peck AB, Cornelius JG. Reversal of insulin-dependent diabetes using islets generated in vitro from pancreatic stem cells. Nat Med 2000. 6(3):278-282. [DOD] [CrossRef]
- Oshima Y, Suzuki A, Kawashimo K, Ishikawa M, Ohkohchi N, Taniguchi H. Isolation of mouse pancreatic ductal progenitor cells expressing CD133 and c-Met by flow cytometric cell sorting. Gastroenterology 2007. 132(2):720-732. [DOD] [CrossRef]
- Lardon J, Corbeil D, Huttner WB, Ling Z, Bouwens L. Stem cell marker prominin-1/AC133 is expressed in duct cells of the adult human pancreas. Pancreas 2008. 36(1):E1-E6. [DOD] [CrossRef]
- Mato E, Lucas M, Petriz J, Gomis R, Novials A. Identification of a pancreatic stellate cell population with properties of progenitor cells: new role for stellate cells in the pancreas. Biochem J 2009. 421(2):181-191. [DOD] [CrossRef]
- Zulewski H, Abraham EJ, Gerlach MJ, Daniel PB, Moritz W, Muller B, Vallejo M, Thomas MK, Habener JF. Multipotential nestin-positive stem cells isolated from adult pancreatic islets differentiate ex vivo into pancreatic endocrine, exocrine, and hepatic phenotypes. Diabetes 2001. 50(3):521-533. [DOD] [CrossRef]
- Bonner-Weir S, Taneja M, Weir GC, Tatarkiewicz K, Song KH, Sharma A, O'Neil JJ. In vitro cultivation of human islets from expanded ductal tissue. Proc Natl Acad Sci U S A 2000. 97(14):7999-8004. [DOD] [CrossRef]
- Gao R, Ustinov J, Korsgren O, Otonkoski T. In vitro neogenesis of human islets reflects the plasticity of differentiated human pancreatic cells. Diabetologia 2005. 48(11):2296-2304. [DOD] [CrossRef]
- Suarez-Pinzon WL, Lakey JR, Brand SJ, Rabinovitch A. Combination therapy with epidermal growth factor and gastrin induces neogenesis of human islet beta-cells from pancreatic duct cells and an increase in functional beta-cell mass. J Clin Endocrinol Metab 2005. 90(6):3401-3409. [DOD] [CrossRef]
- Dudek RW, Lawrence IE Jr, Hill RS, Johnson RC. Induction of islet cytodifferentiation by fetal mesenchyme in adult pancreatic ductal epithelium. Diabetes 1991. 40(8):1041-1048. [DOD] [CrossRef]
- Hao E, Tyrberg B, Itkin-Ansari P, Lakey JR, Geron I, Monosov EZ, Barcova M, Mercola M, Levine F. Beta-cell differentiation from nonendocrine epithelial cells of the adult human pancreas. Nat Med 2006. 12(3):310-316. [DOD] [CrossRef]
- Yatoh S, Dodge R, Akashi T, Omer A, Sharma A, Weir GC, Bonner-Weir S. Differentiation of affinity-purified human pancreatic duct cells to beta-cells. Diabetes 2007. 56(7):1802-1809. [DOD] [CrossRef]
- Eguchi G. Cellular and molecular background of wolffian lens regeneration. Cell Differ Dev 1988. 25(Suppl):147-158. [DOD] [CrossRef]
- Okada TS. Cellular metaplasia or transdifferentiation as a model for retinal cell differentiation. Curr Top Dev Biol 1980. 16:349-380. [DOD] [CrossRef]
- Schmid V, Alder H. The potential for transdifferentiation of differentiated medusa tissues in vitro. Curr Top Dev Biol 1986. 20:117-135. [DOD] [CrossRef]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006. 126(4):663-676. [DOD] [CrossRef]
- Yamanaka S. Induction of pluripotent stem cells from mouse fibroblasts by four transcription factors. Cell Prolif 2008. 41(Suppl 1):51-56. [DOD]
- Yu J, Hu K, Smuga-Otto K, Tian S, Stewart R, Slukvin II, Thomson JA. Human induced pluripotent stem cells free of vector and transgene sequences. Science 2009. 324(5928):797-801. [DOD] [CrossRef]
- Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature 2010. 463(7284):1035-1041. [DOD] [CrossRef]
- Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature 2008. 455(7213):627-632. [DOD] [CrossRef]
- Baeyens L, De Breuck S, Lardon J, Mfopou JK, Rooman I, Bouwens L. In vitro generation of insulin-producing beta cells from adult exocrine pancreatic cells. Diabetologia 2005. 48(1):49-57. [DOD] [CrossRef]
- Minami K, Okuno M, Miyawaki K, Okumachi A, Ishizaki K, Oyama K, Kawaguchi M, Ishizuka N, Iwanaga T, Seino S. Lineage tracing and characterization of insulin-secreting cells generated from adult pancreatic acinar cells. Proc Natl Acad Sci U S A 2005. 102(42):15116-15121. [DOD] [CrossRef]
- Okuno M, Minami K, Okumachi A, Miyawaki K, Yokoi N, Toyokuni S, Seino S. Generation of insulin-secreting cells from pancreatic acinar cells of animal models of type 1 diabetes. Am J Physiol Endocrinol Metab 2007. 292(1):E158-E165. [DOD] [CrossRef]
- Rooman I, Heremans Y, Heimberg H, Bouwens L. Modulation of rat pancreatic acinoductal transdifferentiation and expression of PDX-1 in vitro. Diabetologia 2000. 43(7):907-914. [DOD] [CrossRef]
- Means AL, Meszoely IM, Suzuki K, Miyamoto Y, Rustgi AK, Coffey RJ Jr, Wright CV, Stoffers DA, Leach SD. Pancreatic epithelial plasticity mediated by acinar cell transdifferentiation and generation of nestin-positive intermediates. Development 2005. 132(16):3767-3776. [DOD] [CrossRef]
- Lardon J, De Breuck S, Rooman I, Van Lommel L, Kruhoffer M, Orntoft T, Schuit F, Bouwens L. Plasticity in the adult rat pancreas: transdifferentiation of exocrine to hepatocyte-like cells in primary culture. Hepatology 2004. 39(6):1499-1507. [DOD] [CrossRef]
- Wu SY, Hsieh CC, Wu RR, Susanto J, Liu TT, Shen CR, Chen Y, Su CC, Chang FP, Chang HM, et al. Differentiation of pancreatic acinar cells to hepatocytes requires an intermediate cell type. Gastroenterology 2010. 138(7):2519-2530. [DOD] [CrossRef]
- Strobel O, Dor Y, Alsina J, Stirman A, Lauwers G, Trainor A, Castillo CF, Warshaw AL, Thayer SP. In vivo lineage tracing defines the role of acinar-to-ductal transdifferentiation in inflammatory ductal metaplasia. Gastroenterology 2007. 133(6):1999-2009. [DOD] [CrossRef]
- De La O JP, Emerson LL, Goodman JL, Froebe SC, Illum BE, Curtis AB, Murtaugh LC. Notch and Kras reprogram pancreatic acinar cells to ductal intraepithelial neoplasia. Proc Natl Acad Sci U S A 2008. 105(48):18907-18912. [DOD] [CrossRef]
- Guerra C, Schuhmacher AJ, Canamero M, Grippo PJ, Verdaguer L, Perez-Gallego L, Dubus P, Sandgren EP, Barbacid M. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell 2007. 11(3):291-302. [DOD] [CrossRef]
- Habbe N, Shi G, Meguid RA, Fendrich V, Esni F, Chen H, Feldmann G, Stoffers DA, Konieczny SF, Leach SD, et al. Spontaneous induction of murine pancreatic intraepithelial neoplasia (mPanIN) by acinar cell targeting of oncogenic Kras in adult mice. Proc Natl Acad Sci U S A 2008. 105(48):18913-18918. [DOD] [CrossRef]
- Morris JP, Cano DA, Sekine S, Wang SC, Hebrok M. Beta-catenin blocks Kras-dependent reprogramming of acini into pancreatic cancer precursor lesions in mice. J Clin Invest 2010. 120(2):508-520. [DOD] [CrossRef]
- Shi G, Zhu L, Sun Y, Bettencourt R, Damsz B, Hruban RH, Konieczny SF. Loss of the acinar-restricted transcription factor Mist1 accelerates Kras-induced pancreatic intraepithelial neoplasia. Gastroenterology 2009. 136(4):1368-1378. [DOD] [CrossRef]
- Bonal C, Thorel F, Ait-Lounis A, Reith W, Trumpp A, Herrera PL. Pancreatic inactivation of c-Myc decreases acinar mass and transdifferentiates acinar cells into adipocytes in mice. Gastroenterology 2009. 136(1):309-319. [DOD] [CrossRef]
- Thorel F, Nepote V, Avril I, Kohno K, Desgraz R, Chera S, Herrera PL. Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature 2010. 464(7292):1149-1154. [DOD] [CrossRef]
- Chung CH, Hao E, Piran R, Keinan E, Levine F. Pancreatic beta-cell neogenesis by direct conversion from mature alpha-cells. Stem Cells 2010. In press. [DOD]
- Chung CH, Levine F. Adult pancreatic alpha-cells: a new source of cells for beta-cell regeneration. Rev Diabet Stud 2010. 7(2):124-131. This issue. [DOD] [CrossRef]
- Aviv V, Meivar-Levy I, Rachmut IH, Rubinek T, Mor E, Ferber S. Exendin-4 promotes liver cell proliferation and enhances the PDX-1-induced liver to pancreas transdifferentiation process. J Biol Chem 2009. 284(48):33509-33520. [DOD] [CrossRef]
- Ferber S, Halkin A, Cohen H, Ber I, Einav Y, Goldberg I, Barshack I, Seijffers R, Kopolovic J, Kaiser N, et al. Pancreatic and duodenal homeobox gene 1 induces expression of insulin genes in liver and ameliorates streptozotocin-induced hyperglycemia. Nat Med 2000. 6(5):568-572. [DOD] [CrossRef]
- Kojima H, Fujimiya M, Matsumura K, Younan P, Imaeda H, Maeda M, Chan L. NeuroD-betacellulin gene therapy induces islet neogenesis in the liver and reverses diabetes in mice. Nat Med 2003. 9(5):596-603. [DOD] [CrossRef]
- Yatoh S, Akashi T, Chan PP, Kaneto H, Sharma A, Bonner-Weir S, Weir GC. NeuroD and reaggregation induce beta-cell specific gene expression in cultured hepatocytes. Diabetes Metab Res Rev 2007. 23(3):239-249. [DOD] [CrossRef]
- Yechoor V, Liu V, Espiritu C, Paul A, Oka K, Kojima H, Chan L. Neurogenin3 is sufficient for transdetermination of hepatic progenitor cells into neo-islets in vivo but not transdifferentiation of hepatocytes. Dev Cell 2009. 16(3):358-373. [DOD] [CrossRef]
- Zalzman M, Gupta S, Giri RK, Berkovich I, Sappal BS, Karnieli O, Zern MA, Fleischer N, Efrat S. Reversal of hyperglycemia in mice by using human expandable insulin-producing cells differentiated from fetal liver progenitor cells. Proc Natl Acad Sci U S A 2003. 100(12):7253-7258. [DOD] [CrossRef]
- Karnieli O, Izhar-Prato Y, Bulvik S, Efrat S. Generation of insulin-producing cells from human bone marrow mesenchymal stem cells by genetic manipulation. Stem Cells 2007. 25(11):2837-2844. [DOD] [CrossRef]
- Fotino C, Ricordi C, Lauriola V, Alejandro R, Pileggi A. Use of bone marrow-derived stem cells for transplantation in diabetes patients. Rev Diabet Stud 2010. 7(2):144-157. This issue. [DOD] [CrossRef]
- Li Y, Zhang R, Qiao H, Zhang H, Wang Y, Yuan H, Liu Q, Liu D, Chen L, Pei X. Generation of insulin-producing cells from PDX-1 gene-modified human mesenchymal stem cells. J Cell Physiol 2007. 211(1):36-44. [DOD] [CrossRef]
- Moriscot C, de Fraipont F, Richard MJ, Marchand M, Savatier P, Bosco D, Favrot M, Benhamou PY. Human bone marrow mesenchymal stem cells can express insulin and key transcription factors of the endocrine pancreas developmental pathway upon genetic and/or microenvironmental manipulation in vitro. Stem Cells 2005. 23(4):594-603. [DOD] [CrossRef]
- Oh SH, Muzzonigro TM, Bae SH, LaPlante JM, Hatch HM, Petersen BE. Adult bone marrow-derived cells trans-differentiating into insulin-producing cells for the treatment of type I diabetes. Lab Invest 2004. 84(5):607-617. [DOD] [CrossRef]
- Tang DQ, Cao LZ, Burkhardt BR, Xia CQ, Litherland SA, Atkinson MA, Yang LJ. In vivo and in vitro characterization of insulin-producing cells obtained from murine bone marrow. Diabetes 2004. 53(7):1721-1732. [DOD] [CrossRef]
- Xie QP, Huang H, Xu B, Dong X, Gao SL, Zhang B, Wu YL. Human bone marrow mesenchymal stem cells differentiate into insulin-producing cells upon microenvironmental manipulation in vitro. Differentiation 2009. 77(5):483-491. [DOD] [CrossRef]
- Suzuki A, Nakauchi H, Taniguchi H. Glucagon-like peptide 1 (1-37) converts intestinal epithelial cells into insulin-producing cells. Proc Natl Acad Sci U S A 2003. 100(9):5034-5039. [DOD] [CrossRef]
- Hori Y, Gu X, Xie X, Kim SK. Differentiation of insulin-producing cells from human neural progenitor cells. PLoS Med 2005. 2(4):e103. [DOD] [CrossRef]
- Santamaria P, Rodríguez-Piza I, Clemente-Casares X, Yamanouchi J, Mulero-Perez L, Aasen T, Raya A, Izpisua Belmonte JC. Turning human epidermis into pancreatic endoderm. Rev Diabet Stud 2010. 7(2):158-167. This issue. [DOD] [CrossRef]
- Hisatomi Y, Okumura K, Nakamura K, Matsumoto S, Satoh A, Nagano K, Yamamoto T, Endo F. Flow cytometric isolation of endodermal progenitors from mouse salivary gland differentiate into hepatic and pancreatic lineages. Hepatology 2004. 39(3):667-675. [DOD] [CrossRef]
- Tateishi K, He J, Taranova O, Liang G, D'Alessio AC, Zhang Y. Generation of insulin-secreting islet-like clusters from human skin fibroblasts. J Biol Chem 2008. 283(46):31601-31607. [DOD] [CrossRef]
- Efrat S. Ex-vivo expansion of adult human pancreatic beta-cells. Rev Diabet Stud 2008. 5(2):116-122. [DOD] [CrossRef]
- Jones PM, Courtney ML, Burns CJ, Persaud SJ. Cell-based treatments for diabetes. Drug Discov Today 2008. 13(19-20):888-893. [DOD] [CrossRef]
- Limbert C, Path G, Jakob F, Seufert J. Beta-cell replacement and regeneration: Strategies of cell-based therapy for type 1 diabetes mellitus. Diabetes Res Clin Pract 2008. 79(3):389-399. [DOD] [CrossRef]
- Check E. Stem cells: the hard copy. Nature 2007. 446(7135):485-486. [DOD] [CrossRef]
- Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, van Es JH, Abo A, Kujala P, Peters PJ, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 2009. 459(7244):262-265. [DOD] [CrossRef]
This article has been cited by other articles:

|
Concise review: clinical programs of stem cell therapies for liver and pancreas
Lanzoni G, Oikawa T, Wang Y, Cui CB, Carpino G, Cardinale V, Gerber D, Gabriel M, Dominguez-Bendala J, Furth ME, Gaudio E, Alvaro D, Inverardi L, Reid LM
Stem Cells 2013. 31(10):2047-2060
|
|
|
The use of stem cells for pancreatic regeneration in diabetes mellitus
Bouwens L, Houbracken I, Mfopou JK
Nat Rev Endocrinol 2013. 9(10):598-606
|
|
|
Research progress of stem cell therapy for type 1 diabetes mellitus
Hu MJ, Pan XH, Pang RQ, Cai XM
Chin J Tissue Engin Res 2012. 16(23):4349-4353
|
|
|
Cancer Stem Cells, EMT, and Developmental Pathway Activation in Pancreatic Tumors
Hindriksen S, Bijlsma MF
Cancers (Basel) 2012. 4(4):989-1035
|
|
|
Identifying thyroid stem/progenitor cells: advances and limitations
Fierabracci A
J Endocrinol 2011. In press
|
|

|
Adult pancreatic alpha-cells: a new source of cells for beta-cell regeneration
Chung CH, Levine F
Rev Diabet Stud 2010. 7(2):124-131
|
|
|