Original Data
| Rev Diabet Stud,
2005,
2(2):70-74 |
DOI 10.1900/RDS.2005.2.70 |
Manganese Superoxide Dismutase Alanine to Valine Polymorphism and Risk of Neuropathy and Nephropathy in Egyptian Type 1 Diabetic Patients
Tarek M. El Masry1, Mona A. M. Abou Zahra1, Mohammed M. El Tawil2, Rawhia A. Khalifa3
1Department of Clinical Pathology, Ain Shams University Hospital, Cairo, Egypt.
2Department of Pediatrics, Faculty of Medicine, Ain Shams University, Cairo, Egypt.
3Department of Clinical Pathology, Research Institute of Ophthalmology.
Address correspondence to: Tarek M. El Masry, e-mail: tarek_msry@yahoo.com.
Keywords: antioxidants, type 1 diabetes, microangiopathy, SOD2 gene, neuropathy
Abstract
Oxidative stress, characterized by a marked increase in the level of oxygen free radicals (OFR), has been implicated in the development of diabetic microangiopathic complications, such as diabetic neuropathy (DN) and nephropathy (DP). Antioxidant enzymes may protect against the rapid onset and progression of microangiopathy, by reducing the excess of OFR and peroxides. Mutations and polymorphisms in genes encoding such enzymes may therefore result in a predisposition to this disorder. AIM: we investigated the role of genes encoding the antioxidant enzyme, mitochondrial superoxide dismutase (Mn-SOD2), in DN and DP pathogenesis in an Egyptian population. We studied Ala(-9)Val polymorphism of the Mn-SOD2 gene in type 1 diabetic patients (n = 65) with DN (n = 40) or DP (n = 45). METHODS: we used polymerase chain reaction (PCR) assays with restriction fragment length polymorphism for rapid detection of polymorphisms. These assays involved the use of mismatch PCR primers to create restriction sites in the amplified product only in presence of the polymorphic base. The PCR product was then digested with AgeI restriction enzyme to detect Ala(-9)Val polymorphic sites. RESULTS: the frequencies of the Ala allele (odds ratio (OR) = 0.438, 95% CI of 0.247 - 0.778) and the Ala/Ala genotype (OR = 0.26, 95% CI of 1.39 - 10.266) were significantly lower in diabetic neuropathy patients. In contrast, the frequencies of the Val allele (OR = 2.282, 95% CI of 1.286 - 4.05) and the homozygous Val/Val genotype (OR = 6.68, 95% CI of 0.3 - 0.76) were significantly higher in patients with DN than diabetics without neuropathy. Although the Val allele was more frequently detected in DP patients than diabetics without nephropathy (OR = 3.2), this difference was statistically non-significant. In conclusion, Ala(-9)Val substitution in the Mn-SOD2 gene was associated with DN in Egyptian diabetic children but not a significant factor in diabetic patients with nephropathy.
Introduction
Diabetes has reached epidemic proportions, affecting more than 6% of the world’s population. The morbidity and mortality of diabetes are due to the development of both macrovascular and microvascular complications. While these macrovascular complications are common among diabetics, diabetes-specific microvascular complications will eventually affect nearly all individuals with diabetes. More than half of all patients with diabetes develop neuropathy. Hyperglycemia is a necessary but not a sufficient factor to account for the development of diabetic nephropathy, whereas hyperglycemia seems to be the major risk factor for proliferative retinopathy. It is hypothesized that diabetic microangiopathy in different organs is genetically heterogeneous. This is because, given sufficient duration, retinopathy is almost universal, but not all patients will develop nephropathy or neuropathy [1].
Oxygen free radicals may damage neurons through lipid peroxidation, resulting in the breakdown of mitochondrial DNA and inhibition of the respiratory chain, which may lead to cross-linking of neurofilament proteins. It has been suggested that production of intracellular antioxidant enzymes in response to high glucose levels may be defective in patients with type 1 diabetes mellitus (DM) with microangiopathy. This defect may be part of the genetic predisposition to microangio-pathic complications. An alanine (GCT) to valine (GTT) substitution at position (-9) in the signal peptide of human Mn-SOD (Ala-9Val) has been shown to change the structural conformation of the mitochondrial targeting sequence of the enzyme. This substitution may lead to misdirected intracellular trafficking, followed by changes in Mn-SOD activity [2]. Associations have been found between alanine/valine dimorphism at position (-9) in the SOD2 gene and human neurodegenerative and aging disorders such as Parkinson’s disease, tardive dyskinesia and non-familial idiopathic cardiomyopathy [3].
Patients and study groups
In this study we investigated the effect and relationship of allelic variants of the Mn-SOD gene in Egyptian type 1 diabetic patients with neuropathy and nephropathy. A simple and rapid polymerase chain reaction with restriction fragment length polymorphism (PCR-RFLP) was used to detect gene polymorphism.
This study included 40 normal healthy controls, 25 males and 15 females. Their mean age in years was 17 ± 6. One hundred and fifty type 1 diabetic patients, selected from the outpatient clinic at Ain-Shams University Hospital, Cairo, Egypt, constituted the study groups. They were further subdivided into three groups:
- Diabetic group without microangiopathy, which included 65 patients, 33 males and 32 females, with a mean age in years of 18 ± 3.5. These patients had no microangiopathic complications.
- Diabetic neuropathy (DN) group, which consisted of 40 patients, 26 males and 14 females, with a mean age in years of 17 ± 4.5. These patients had peripheral neuropathy diagnosed on the basis of symptomatic symmetrical distal neuropathy (reduced or absent ankle reflexes, vibration sense at the medial malleolus and/or reduced sense of position with one or more typical symptoms, such as burning sensation, cramps, paresthesia or numbness).
- Diabetic nephropathy (DP) group, which included 45 patients, 28 males and 17 females, with a mean age in years of 16 ± 4. These diabetic patients had clinical nephropathy, diagnosed on the basis of persistent microalbuminuria, indicating incipient nephropathy. The characteristics of both control and study groups are summarized in Table 1.
Informed consent was obtained from all patients and children’s parents before participation in this study. None of the study patients was treated with antioxidants.
Table
1.
Clinical and laboratory characteristics of type 1 diabetic patients and healthy controls |
|
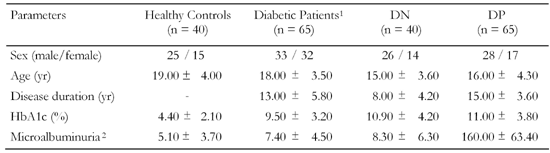 |
 |
Legend:
Data are means ± SD. 1 Without complications. 2 μg/mg urine creatinine. |
|
Study design and methods
DNA extraction and PCR amplification
Genomic DNA was extracted from the buffy coat fraction with phenol and chloroform. Mn-SOD genotypes were determined by the method of polymerase chain reaction (PCR) followed by restriction fragment length polymorphism (RFLP), as reported by Budowle et al. [4]. The primers for Val-9Ala polymorphism were 5'-CCAGCAGGCAGCTGGCACCG-3' (forward) and 5'-TCCAGGGCGCCGTAGTCGTAGG-3' (reverse). The PCR was performed in a Perkin Elmer 4800 thermal cycler after an initial step of denaturation of the reaction mixture at 95°C for 15 minutes, followed by 35 cycles at 94°C for 60 seconds, 60°C for 60 seconds (annealing) and at 72°C for 60 seconds (extension). The PCR was completed by a final extension cycle at 72°C for 7 minutes. For detection of Val-9Ala polymorphism, the PCR product (91 bp) with the C allele (Ala) was digested by restriction enzyme AgeI into two fragments (74 and 17 bp), whereas the PCR product with the T allele (Val) cannot be cut and therefore yielded only a single fragment. Digested DNA fragments were then separated by electrophoresis using 3% agarose gel and visualized by ethidium bromide staining. Molecular weight DNA ΦX174/HinfI was used as a DNA marker.
The original sequence, near the polymorphic site of Mn-SOD, is 5'-TCCGGT-3' with the most variable thymidine (Val) to cystidine (Ala) at position (-9). The first adenosine residue from the 3' end of the forward primer was mismatched with genomic DNA, resulting in a 91 bp PCR product. This PCR may or may not contain the AgeI restriction site (5'-ACCGGT-3'), depending on the sequence. If codon (-9) was Ala (GCT), then the amplified product was not digested, resulting in only one electrophoretic band of 91 bp near to the 100 bp DNA marker on the agarose gel. If the sample was heterozygous for Val (GTT) at codon (-9), then digestion resulted in two DNA fragments, 17 and 74 bp in length. The visualized bands in this sample consisted of two bands, one of 91 bp near to the 100 bp DNA marker and one of 74 bp between the 66 and 82 bp DNA marker, while the 17 bp DNA product was too faint to be visualized on agarose gel. If the sample was homozygous for Val alleles (Val/Val) genotype, digestion resulted in two DNA products, but with only one visualized band of 72 bp between 66 and 82 bp DNA marker. Electrophoretic patterns are shown in Figure 1.
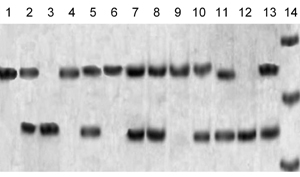 |
 |
Figure 1. Lane 14 is for the DNA marker, showing bands of 66, 82 and 100 bp. Lanes 1, 4, 6, 9 show the Ala/Ala genotype. Lanes 2, 5, 7, 8, 10, 11, and 13 show the Val/Ala genotype. Lanes 3 and 12 show the Val/Val genotype. |
|
Statistical analysis
To evaluate the differences for categorical data, including genotype distribution, the χ2 test using a rows and columns program based on the Bentzen algorithm was used [5]. Genotype and allele frequencies in the study group were compared by Fisher’s exact test. The p-value was corrected by multiplying it by the number of alleles (2) for each locus in order to obtain pc values. Odds ratios (OR) and 95% confidence interval (95% CI) were calculated to assess the strength of the relationship between Mn-SOD polymorphism and DN.
Results
Genotype and allele frequencies for Mn-SOD are presented in Table 2. The Ala alleles and the Val/Ala genotype were the most common in healthy subjects. The observed level of heterozygosity was 45.8%. The frequencies of the Ala allele (OR = 0.438 with 95% CI of 0.247 - 0.778) and the Ala/Ala genotype (OR = 0.26 with 95% CI of 1.39 - 10.266) were significantly lower (p < 0.05) in patients with diabetic neuropathy than in patients without DN. Thus, they are associated with a lower risk of developing DN. Con-versely, the frequency of the Val allele (OR = 2.282 with 95% CI of 1.286 - 4.05) and the homozygous Val/ Val genotype (OR = 6.68 with 95% CI of 0.3 - 0.76) was significantly higher (p < 0.05) in patients with DN. This suggests that the Ala(-9)-Val dimorphism in the Mn-SOD gene is associated with a high risk of the development of neuropathy in type 1 diabetes mellitus. Although the Val allele frequency was greater in DP patients than in patients without DP (OR = 3.2), this difference was statistically non-significant (p > 0.05). The Val genotypes and Ala alleles and genotypes showed no significant abnormalities between diabetic patients without complications and those with DP (p > 0.05).
Table
2.
Frequencies of Mn-SOD alleles and genotypes in the study and the control group |
|
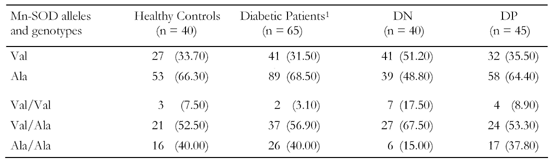 |
 |
Legend:
Data in parentheses are percentage values. 1 Without complications. |
|
Conclusions
Our results showed that the Ala allele of the Mn-SOD gene was more widespread in the general population than the Val allele, and the Val/Ala genotype was the most common in the Egyptian population. These results agree with those of Chistyakov et al., 2001 [6].
Poor signal sequence recognition by a receptor in the inner mitochondrial membrane may result in mistargeting. In addition, inefficient cleavage of a particular signal may reduce the level of enzymatic activity of an imported protein, such as Mn-SOD, within the mitochondrial compartment. Studies of processing have suggested that the basal level of Mn-SOD activity may be highest for Ala/Ala, followed by Val/Ala, and then Val/Val genotypes [7].
In agreement with our results, Strokov et al. showed that Ala(-9)-Val polymorphism in a Russian population was associated with a high risk of the development of neuropathy in type 1 diabetic patients [8]. Nosikov and colleagues concluded that diabetic polyneuropathy is associated with single-nucleotide polymorphism, such as Ala(-9)-Val of the SOD 2 gene, Arg 213-Gly of SOD 3, and T(-262)-C of CAT, and with a polymorphic microsatellite of the nitric oxide synthase gene [9]. In our study, the Val allele and the Val/Val genotype could be detected more frequently in diabetic patients with nephropathy, but the association was statistically non-significant. In contrast, Nomiyama et al. studied Val(16)-Ala polymorphism of Mn-SOD in patients with diabetes and diabetic nephropathy in a Japanese population with type 2 diabetes [10]. They found a significant association between Val(16)-Ala polymorphism of Mn-SOD and the development of nephropathy in those patients. The Val variant of the Mn-SOD may be present in a lower concentration in mitochondria. If this is the case, then patients with Val/Val homozygosity should have lower resistance to oxidative stress than patients with other Mn-SOD variants, which is a common feature in DM and neurological disorders. Our study showed that Val Mn-SOD allele predisposes type 1 diabetic patients to the development of neuropathy. Inefficient targeting of Mn-SOD may leave mitochondria inadequately protected against superoxide radicals. This may lead to protein oxidation, mitochondrial DNA mutation and damage. Recently, Aykol et al. found an association between Val(-9)-Ala polymorphism and schizophrenia, suggesting that this polymorphism may contribute to neurodegenerative disorders [11]. Accordingly, the Mn-SOD gene may not be involved in the pathogenesis of DN, but this mutation is more likely to be involved in neurodegenerative disorders.
Different methods have been proposed for detecting Val(-9)-Ala polymorphism. We choose PCR amplification of the polymorphic region, followed by AgeI treatment (restriction fragment length polymorphism, RFLP). This restriction enzyme is commercially available and this method has advantages over other methods, such as single-strand conformational polymorphism (SSCP) and two-step PCR, which use allele-specific primers for a second round of PCR followed by hybridization of the PCR product with allele-specific oligonucleotide probes. Our molecular assay includes the use of the mismatch-PCR/RFLP approach, which can be applied in order to detect any polymorphic single base substitution that fails to create or eliminates restriction sites. The use of mismatch PCR primer makes it possible to create a restriction site in the amplified product only in the presence of a polymorphic base.
In conclusion, we found that the Mn-SOD Val/Ala polymorphism and the Val allele were associated with an increased risk of the development of neuropathy but not nephropathy in patients with type 1 DM. However, there was a slight increase of Val allele in nephropathic patients, which needs further evaluation. Finally, we have no comparative data concerning the role of genetic factors being directly related to oxidative stress in Egyptian DN and DP initiation and progression. It would be of value to compare our findings with those of similar association studies in other Egyptian populations.
References
- Svensson M, Eriksson JW, Dahlquist G. Early glycemic control, age at onset, and development of microvascular complications in childhood-onset type 1 diabetes: a population-based study in northern Sweden. Diabetes Care 2004. 27:955-962. [DOD]
- Rosenblum JS, Gilula NB, Lerner RA. On signal sequence polymorphisms and diseases of distribution. Proc Natl Acad Sci U S A 1996. 93:4471-4473. [DOD] [CrossRef]
- Hori H, Ohmori O, Shinkai T, Kojima H, Okano C, Suzuki T, Nakamura J. Manganese superoxide dismutase gene polymorphism and schizophrenia: relation to tardive dyskinesia. Neuropsychopharmacology 2000. 23:170-177. [DOD] [CrossRef]
- Budowle B, Chakraborty R, Giusti AM, Eisenberg AJ, Allen RC. Analysis of the VNTR locus D1S80 by the PCR followed by high-resolution PAGE. Am J Hum Genet 1991. 48:137-144. [DOD]
- Roff DA, Bentzen P. The statistical analysis of mitochondrial DNA polymorphisms: chi 2 and the problem of small samples. Mol Biol Evol 1989. 6:539-545. [DOD]
- Chistyakov DA, Savost’anov KV, Zotova EV, Nosikov VV. Polymorphisms in the Mn-SOD and EC-SOD genes and their relationship to diabetic neuropathy in type 1 diabetes mellitus. BMC Med Genet 2001. 2:4. [DOD] [CrossRef]
- Grasbon-Frodl EM, Kosel S, Riess O, Muller U, Mehraein P, Graeber MB. Analysis of mitochondrial targeting sequence and coding region polymorphisms of the manganese superoxide dismutase gene in German Parkinson disease patients. Biochem Biophys Res Commun 1999. 255:749-752. [DOD] [CrossRef]
- Strokov IA, Bursa TR, Drepa OI, Zotova EV, Nosikov VV, Ametov AS. Predisposing genetic factors for diabetic polyneuropathy in patients with type 1 diabetes: a population-based case-control study. Acta Diabetol 2003. 40(Suppl 2):S375-379. [DOD] [CrossRef]
- Nosikov VV. Genomics of type I diabetes mellitus and its late complications. Mol Biol (Mosk) 2004. 38:150-164. [DOD]
- Nomiyama T, Tanaka Y, Piao L, Nagasaka K, Sakai K, Ogihara T, Nakajima K, Watada H, Kawamori R. The polymorphism of manganese superoxide dismutase is associated with diabetic nephropathy in Japanese type 2 diabetic patients. J Hum Genet 2003. 48:138-141. [DOD]
- Akyol O, Yanik M, Elyas H, Namli M, Canatan H, Akin H, Yuce H, Yilmaz HR, Tutkun H, Sogut S, Herken H, Ozyurt H, Savas HA, Zoroglu SS. Association between Ala-9Val polymorphism of Mn-SOD gene and schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 2005. 29:123-131. [DOD] [CrossRef]
This article has been cited by other articles:

|
Association of the C47T polymorphism in SOD2 with diabetes mellitus and diabetic microvascular complications: a meta-analysis
Tian C, Fang S, Du X, Jia C
Diabetologia 2011. 54(4):803-811
|
|
|
The sequence polymorphism of MnSOD gene in subjects with respiratory insufficiency in COPD
Pietras T, Szemraj J, Witusik A, Holub M, Panek M, Wujcik R, Gorski P
Med Sci Monit 2010. 16(9):CR427-CR432
|
|

|
Association between the rs4880 superoxide dismutase 2 (C>T) gene variant and coronary heart disease in diabetes mellitus
Jones DA, Prior SL, Tang TS, Bain SC, Hurel SJ, Humphries SE, Stephens JW
Diabetes Res Clin Pract 2010. 90(2):196-201
|
|
|
Association between SOD2 T-9C and MTHFR C677T polymorphisms and longevity: a study in Jordanian population
Khabour OF, Abdelhalim ES, Abu-Wardeh A
BMC Geriatr 2009. 9:57
|
|

|
Age-dependent dichotomous effect of superoxide dismutase Ala16Val polymorphism on oxidized LDL levels
Dedoussis GV, Kanoni S, Panagiotakos DB, Louizou E, Grigoriou E, Chrysohoou C, Pitsavos C, Stefanadis C
Exp Mol Med 2008. 40(1):27-34
|
|
|