Chapter III. Diabetic Retinopathy
| Rev Diabet Stud,
2015,
12(1-2):196-210 |
DOI 10.1900/RDS.2015.12.196 |
New Therapeutic Approaches in Diabetic Retinopathy
Kamyar Vaziri, Stephen G. Schwartz, Nidhi Relhan, Krishna S. Kishor, Harry W. Flynn Jr
Department of Ophthalmology, Bascom Palmer Eye Institute, University of Miami Miller School of Medicine, 900 NW 17th Street, Miami, FL 33136, USA
Address correspondence to: Stephen G. Schwartz, MD, MBA, Bascom Palmer Eye Institute, 3880 Tamiami Trail North, Naples, FL 34103, e-mail: sschwartz2@med.miami.edu
Manuscript submitted April 24, 2015; accepted April 30, 2015.
Keywords: diabetic retinopathy, macular edema, proliferative diabetic retinopathy, vascular endothelial growth factor
Abstract
Diabetic retinopathy is a common microvascular complication of diabetes mellitus. It affects a substantial proportion of US adults over age 40. The condition is a leading cause of visual loss. Much attention has been given to expanding the role of current treatments along with investigating various novel therapies and drug delivery methods. In the treatment of diabetic macular edema (DME), intravitreal pharmacotherapies, especially anti-vascular endothelial growth factor (anti-VEGF) agents, have gained popularity. Currently, anti-VEGF agents are often used as first-line agents in center-involved DME, with recent data suggesting that among these agents, aflibercept leads to better visual outcomes in patients with worse baseline visual acuities. While photocoagulation remains the standard treatment for proliferative diabetic retinopathy (PDR), recent FDA approvals of ranibizumab and aflibercept in the management of diabetic retinopathy associated with DME may suggest a potential for pharmacologic treatments of PDR as well. Novel therapies, including small interfering RNAs, chemokines, kallikrein-kinin inhibitors, and various anti-angiogenic agents, are currently being evaluated for the management of diabetic retinopathy and DME. In addition to these strategies, novel drug delivery methods such as sustained-release implants and refillable reservoir implants are either under active evaluation or have recently gained FDA approval. This review provides an update on the novel developments in the treatment of diabetic retinopathy.
Abbreviations: ACCORD – Action to Control Cardiovascular Risk in Diabetes; CCL-2 – chemokine ligand 2; CSME – clinically significant macular edema; DA VINCI – DME and VEGF Trap-Eye: Investigation of Clinical Impact; DCCT – Diabetes Control and Complications Trial; DEGAS – Dose-Ranging Evaluation of Intravitreal siRNA PF-04523655 for DME; DME – diabetic macular edema; DRCR – Diabetic Retinopathy Clinical Research Network; DRS – Diabetic Retinopathy Study; DRSSW – Diabetic Retinopathy Screening Service for Wales; ECT – encapsulated cell technology; ETDRS – Early Treatment Diabetic Retinopathy Study; FAME – Fluocinolone Acetonide for Diabetic Macular Edema; FDA – Food and Drug Administration; FIELD – Fenofibrate Intervention and Event Lowering in Diabetes; GAG – glycosaminoglycan; HbA1c – glycosylated hemoglobin; HDL – high-density lipoprotein; IOP – intraocular pressure; IVTA – intravitreal triamcinolone acetonide; LDL – low-density lipoprotein; MEAD – Macular Edema: Assessment of Implantable Dexamethasone in Diabetes; mTOR – mammalian target of rapamycin; NPDR – non-proliferative diabetic retinopathy; OCT – optical coherence tomography; PAI – platelet aggregation inhibitor; PDR – proliferative diabetic retinopathy; PRP – panretinal photocoagulation; RIDE – Ranibizumab Injection in Subjects With Center Involvement Secondary to Diabetes Mellitus; RISE – Ranibizumab Injection in Subjects With Clinically Significant Macular Edema; siRNA – small interfering RNA; TIE2 – tunica internal endothelial cell kinase 2; VEGF – vascular endothelial growth factor; VIVID-DME – Intravitreal Alfibercept Injection in Vision Impairment Due to DME; VISTA-DME – Study of Intravitreal Administration of VEGF Trap-Eye in Patients with Diabetic Macular Edema; UKPDS – UK Prospective Diabetes Study
1. Background
1.1 Epidemiology
Over 29 million people in the US, or 9.3% of the population, had diabetes mellitus in 2012 [1]. An estimated prevalence of 28.5% among US adults with diabetes, or 3.8% of all US adults aged 40 years or older, had diabetic retinopathy between 2005 and 2008 [2]. In the US population, 1.5% of diabetic adults aged 40 and older are affected by proliferative diabetic retinopathy (PDR) and 2.7% by clinically significant macular edema (CSME) [3]. Globally, it is estimated that there were approximately 93 million people, or 35% of diabetic adults aged 20-76 years, with any diabetic retinopathy, 17 million people with PDR, and 21 million people with diabetic macular edema (DME) in 2010 [3].
Diabetic retinopathy seems to be more common in men than in women in the US [2], although there are no reported significant gender differences worldwide [3]. Diabetic retinopathy can affect individuals from all racial and ethnic backgrounds. However, it has been reported that African-Americans and Hispanics in the US have higher rates of both diabetic retinopathy and sight-threatening diabetic retinopathy than non-Hispanic whites [2, 4, 5]. Data from other regions of the world suggest that African/Afro-Caribbean, South Asian, Latin American, and indigenous tribal populations tend to have a higher prevalence of diabetic retinopathy [4, 6].
Diabetic retinopathy has been reported more commonly associated with type 1 than with type 2 diabetes mellitus. A recent study utilizing the data from the Diabetic Retinopathy Screening Service for Wales (DRSSW) reported a prevalence of diabetic retinopathy of 56% in type 1 diabetes patients and 30% in type 2 diabetes patients. Similarly, the prevalence of sight-threatening diabetic retinopathy was higher in type 1 (11%) than in type 2 diabetes patients (3%) [7].
1.2 Risk factors
Numerous risk factors have been associated with diabetic retinopathy, including duration of diabetes, high HbA1c levels (chronic hyperglycemia), hypertension, and ethnicity [3, 8-12]. Potential risk factors such as dyslipidemia and body mass index (or obesity) have been less consistently linked with diabetic retinopathy; some studies have reported these as risk factors [13-16], while others have not [5, 17-19].
1.3 Diabetic retinopathy projections and need for treatment
It is estimated that by 2035, 592 million people worldwide will have diabetes [20]. In the US, the number of individuals with diabetic retinopathy is projected to approximately double by 2050 to over 15 million, from over 7 million people in 2010 [21]. The number of individuals with vision-threatening diabetic retinopathy is projected to increase from 1.2 million in 2005 to close to 2.2 million people in 2020 [21]. After 10 years of follow-up, a reported 53% of patients with non-proliferative diabetic retinopathy (NPDR) at baseline developed pre-proliferative diabetic retinopathy and 11% developed PDR. Among diabetic patients with no retinopathy at baseline, 66% developed NPDR at 10 years and 1.5% developed PDR at 10 years [22].
2. Current treatment
2.1 Control of modifiable risk factors
The Diabetes Control and Complications Trial (DCCT) reported that patients with insulin-dependent diabetes mellitus receiving 'intensive' treatments aimed at tight glycemic control had a 74% reduction in the risk of developing diabetic retinopathy at a mean of 6.5 years of follow-up when compared with patients receiving conventional therapy [12]. In a follow-up study of the same DCCT participants with type 1 diabetes, the 'intensive' therapy group maintained a reduced risk of developing diabetic retinopathy after 15-18 years, but the relative difference was smaller than in the original study [23].
Hypertension is another modifiable risk factor that has been shown to influence the development and progression of diabetic retinopathy. The UK Prospective Diabetes Study (UKPDS) reported that, among type 2 patients with diabetic retinopathy, tight control of blood pressure resulted in a 47% reduction in the risk of visual acuity loss of 3 or more lines [11]. A 2015 review of qualifying randomized clinical trials on the effect of blood pressure on diabetic retinopathy reported that overall, intensive blood pressure control reduced the 4- to 5-year odds of developing diabetic retinopathy (estimated risk ratio = 0.78) [24]. However, a consistent benefit of blood pressure control in progression to PDR or development of CSME was not shown.
The Action to Control Cardiovascular Risk in Diabetes (ACCORD) eye study reported its 4-year results on the effect of intensive blood pressure control (target systolic blood pressure <120) or intensive glycemic control (target HbA1c <6.0%) on diabetic retinopathy. Among diabetic patients with no diabetic retinopathy at baseline, neither intensive glycemic control nor intensive blood pressure control had a significant effect on the development of any diabetic retinopathy stage. Among patients with diabetic retinopathy at baseline, however, intensive glycemic control significantly reduced the odds of ≥1-, ≥2- or ≥3-step worsening of diabetic retinopathy on the Early Treatment Diabetic Retinopathy Study (ETDRS) scale, with the strongest effect among eyes with microaneurysms only or mild NPDR. In this study, intensive blood pressure control did not show any benefits in the development or progression of diabetic retinopathy [25].
Fenofibrate is a medication sometimes prescribed to reduce low-density lipoprotein (LDL) and increase high-density lipoprotein (HDL) levels. In addition to its role in the management of lipids, fenofibrate is reported to have anti-inflammatory, anti-angiogenic, anti-apoptotic, and antioxidant properties [26]. The Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study including 1,012 patients with type 2 diabetes reported that a significantly smaller proportion of patients in the fenofibrate treatment group received photocoagulation when compared with the group not receiving fenofibrate. Furthermore, the proportion of participants in the treatment group with diabetic retinopathy at baseline, who had a 2-step progression based on ETDRS scale, was significantly smaller than the proportion of those participants in the group without fenofibrate treatment (3.1% vs. 14.6% respectively; p = 0.004) [27]. More recent results from the ACCORD study reported that patients receiving this medication were less likely to have ≥3- and ≥4-step diabetic retinopathy progression [25]. Despite these clinical results, fenofibrate is not commonly used for the purpose of reducing the progression of diabetic retinopathy.
2.2 Photocoagulation
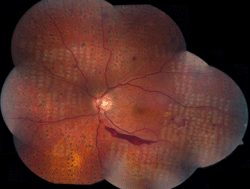
Panretinal photocoagulation (PRP) involves the placement of photocoagulation treatment in the peripheral retina in order to induce regression of abnormal neovascular tissue. The Diabetic Retinopathy Study (DRS) was a randomized controlled trial evaluating the effects of PRP versus observation on more than 1,700 participants with severe NPDR (in both eyes) or PDR (in at least one eye) (Figure 1). Compared to the untreated study eyes, the DRS reported an approximate 50% reduction in the incidence of severe visual loss in the PRP-treated eyes throughout the 5-year study period. This reduced incidence rate at 5 years was apparent in both NDPR and PDR groups, with the “high-risk” PDR group benefiting the most (57% reduction in severe visual loss) [28].
 |
 |
Figure 1. Montage fundus photography of left eye, demonstrating proliferative diabetic retinopathy and panretinal photocoagulation burns. The white burns are fresh (placed approximately one hour prior to obtaining the photograph). The pigmented burns are several weeks older. |
|
The ETDRS evaluated the benefit of early treatment with PRP among 3,711 participants with mild to severe NPDR or early PDR. The reported 5-year rates of severe visual loss were small in both the treatment (2.6%) and control (deferred treatment) groups (3.7%), therefore PRP was not recommended for these patients [29]. A follow-up study evaluating the visual acuity of the remaining ETDRS participants after a median of 16.7 years after the initial PRP showed that most of those patients retained good vision with 84% having at least 20/40 visual acuity in the better eye [25].
A review of qualifying randomized controlled trials evaluated the overall effect of PRP in PDR (including 9,503 eyes) and reported that photocoagulation appeared to reduce the risk of severe visual loss by 50%, and reduced the risk of both retinopathy progression and vitreous hemorrhage, also by approximately 50% [30].
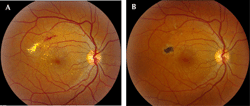
The ETDRS studied the benefits of focal/grid photocoagulation versus observation in 2,244 participants with CSME and mild to moderate NPDR. It was reported that the treatment group experienced a 50% reduction in the risk of moderate vision loss, whereby eyes with center-involved macular edema benefitted most [31] (Figure 2). However, in this study, fewer than 3% of eyes with CSME experienced a visual gain of 15 letters of more. Since two-thirds of the eyes in the ETDRS study had 20/25 or better at baseline, the ability to improve visual acuity was necessarily limited.
 |
 |
Figure 2. Fundus photography of diabetic macular edema. A. Fundus photography of right eye, demonstrating diabetic macular edema. B. Fundus photography of the same eye several months later following focal/grid photocoagulation. The diabetic macular edema has improved. |
|
2.3 Corticosteroids
Intravitreal injections of corticosteroids for the treatment of DME have been extensively studied. Three synthetic corticosteroids (triamcinolone acetonide, dexamethasone, and fluocinolone) have been evaluated. All intravitreal injections of corticosteroids were associated with risks of endophthalmitis, retinal tear/detachment, vitreous hemorrhage, elevated intraocular pressure (IOP), and cataract [32, 33].
Triamcinolone acetonide. In 2008, the Diabetic Retinopathy Clinical Research Network (DRCR) conducted a randomized controlled trial comparing focal/grid photocoagulation with 1 mg and 4 mg intravitreal triamcinolone acetonide (IVTA), an off-label therapy (DRCR protocol B). At 4 months, the 4 mg treatment group showed better mean visual acuity than the other 2 groups, but at 12 months, there were no significant differences among the three groups. At 16 months, both the 1 mg and 4 mg IVTA group had significantly worse mean visual acuities than the photocoagulation group. Furthermore, the IVTA groups had much higher rates of increased IOP and cataract [35]. In 2010, the DRCR-reported protocol I, a randomized controlled trial comparing focal/grid photocoagulation alone or with combined intravitreal injection of ranibizumab (Lucentis, Genentech, South San Francisco, CA) or IVTA in patients with center-involved DME [35]. At 1 year, combined IVTA and photocoagulation significantly reduced the central subfield thickness evaluated by optical coherence tomography (OCT), but there were no significant improvements in visual acuity in comparison with photocoagulation alone. In the pseudophakic subgroups, however, combined IVTA and photocoagulation was associated with significantly better visual acuities than photocoagulation alone. In summary, the results of combined IVTA and photocoagulation remained inferior to ranibizumab with or without photocoagulation.
A 1-year study, comparing IVTA combined with PRP versus PRP alone in eyes with both PDR and DME, reported significantly better visual gains along with significant improvement in OCT parameters in the combined PRP/IVTA treatment group [36]. Similar positive results with combination of IVTA and PRP in eyes with PDR and DME have been reported by other studies [37, 38]. An exploratory analysis of the DRCR study on DME management showed that patients with PDR at baseline had a significantly reduced risk of worsening retinopathy when treated with both IVTA and PRP when compared with the PRP alone [39]. This study reported similar reductions in retinopathy progression with ranibizumab and either prompt or deferred photocoagulation. However, despite these positive clinical trial results, IVTA is not FDA-approved for the treatment of DME or PDR in the US.
Dexamethasone. A bioerodable intravitreal dexamethasone implant (Ozurdex, Allergan, Irvine, CA) has been approved for the treatment of DME. In two randomized controlled trials, this implant was associated with improved visual acuity. In one study, a significantly greater proportion of patients receiving focal/grid photocoagulation combined with the dexamethasone implant achieved improvements in visual acuity of at least 10 letters at 9 months than that of patients receiving photocoagulation alone. However, the visual acuities of the two groups were not significantly different after 12 months [40].
The Macular Edema: Assessment of Implantable Dexamethasone in Diabetes (MEAD) study reported that treatment with the dexamethasone implant was associated with a higher proportion of eyes achieving at least 15 letters of visual acuity improvement at 3 years compared with sham injections [41]. Increased IOP and cataract rates were the most commonly reported side effects in the treatment groups.
Fluocinolone acetonide has also been studied in the treatment of DME. A surgically implanted non-bioerodable fluocinolone-eluting device, Retisert (Bausch and Lomb, Madison, NJ), is FDA-approved for the treatment of chronic non-infectious posterior segment uveitis. In a 3-year clinical trial, the proportion of subjects gaining ≥3 lines of visual acuity was significantly higher in patients treated with this implant after 6, 9, and 24 months, but not after 3 years, compared with the "standard of care" control group. Furthermore, the implant group had high rates of cataract (>90%) and elevated IOP (61.4%) at 4 years, with more than 30% of the implanted eyes undergoing incisional surgery because of uncontrolled IOP [42].
A smaller, non-bioerodable fluocinolone acetonide insert that may be injected in a clinical setting (Iluvien, Alimera, Alpharetta, GA) is FDA-approved for the treatment of DME in eyes that previously have been treated with corticosteroids and that did not have a clinically significant rise in IOP. The Fluocinolone Acetonide for Diabetic Macular Edema (FAME) trials reported that the inserts were associated with a greater proportion of eyes achieving 15 or more letters of visual acuity improvement at 24 months compared with sham treatment [43]. An extension of the FAME studies reported that after 3 years, the beneficial effects of the implant persisted [44]. In these trials, almost all treated phakic eyes developed cataract and 4.8% - 8.1% of treated eyes ultimately required incisional glaucoma surgery to control IOP [44].
2.4 Anti-VEGF agents
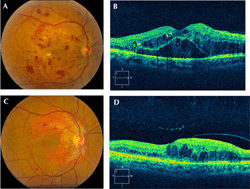
Increased levels of vascular endothelial growth factor (VEGF) in diabetic retinopathy were first reported in the 1990s [45, 46]. Four anti-VEGF agents have been studied in the treatment of diabetic retinopathy (Figure 3): bevacizumab (Avastin, Genentech, South San Francisco, CA), ranibizumab (Lucentis, Genentech, South San Francisco, CA), aflibercept (Eylea, Regeneron, Tarrytown, NY), and pegaptanib (Macugen, Valeant, Madison, NJ).
 |
 |
Figure 3. Fundus photography of diffuse diabetic macular edema. A. Fundus photography of right eye, demonstrating diffuse diabetic macular edema. B. Optical coherence tomography of the same eye, demonstrating cystoid macular edema. C. Fundus photography of the same eye following three years of treatment with anti-vascular endothelial growth factor agents. The diabetic macular edema has improved. D. Optical coherence tomography of the same eye, demonstrating improvement of cystoid macular edema. |
|
Bevacizumab. Off-label use of intravitreal bevacizumab has been used widely in the treatment of DME. Several clinical trials have reported that intravitreal bevacizumab was associated with more favorable outcomes than focal/grid photocoagulation in the treatment of DME [47-49].
Intravitreal injection of bevacizumab has been reported to reduce the regression of neovascularization in eyes treated with PDR [50-53]. It has been applied as an adjunct therapy with PRP [54, 55], and as a pre-operative adjunct therapy with pars plana vitrectomy [56-58].
Ranibizumab. Ranibizumab is FDA-approved for the treatment of DME and diabetic retinopathy associated with DME. The second indication emerged as a result of the phase III randomized controlled trials of ranibizumab for diabetic macular edema (RISE and RIDE). These two industry-sponsored randomized controlled trials evaluated the benefits of 0.3 mg or 0.5 mg monthly intravitreal ranibizumab injections in patients with DME [59]. While the main outcome measure was the proportion of participants gaining ≥15 letters at 2 years, an analysis of patients with both DME and diabetic retinopathy at baseline showed that the groups treated with ranibizumab had lower rates of retinopathy deterioration and higher rates of retinopathy improvement. Also, the ranibizumab treatment groups were less likely to develop PDR. Ranibizumab 0.3 mg monthly injections for DME were approved by the FDA, based on similar efficacy and fewer systemic side effects in the 0.3 mg vs. the 0.5 mg study group.
In multiple randomized controlled trials, ranibizumab has been reported beneficial in the treatment of DME as a monotherapy or as an adjuvant to focal/grid photocoagulation [59-66]. In the RIDE/RISE and DRCR protocol I studies, favorable outcomes were sustained for up to 5 years respectively with continued therapy [67, 68]. Recently, the DRCR (protocol S) found that ranibizumab was non-inferior to PRP in patients with PDR, suggesting that pharmacologic therapy alone may be a reasonable option in some patients with PDR [69].
Pegaptanib. A phase II/III randomized controlled trial reported that a significantly greater proportion of DME patients who received pegaptanib had improvements in visual acuity than of those DME patients receiving sham injections only [70]. A prospective exploratory study compared intravitreal injection of pegaptanib alone with panretinal photocoagulation alone in patients with PDR. It was reported that 100% of eyes treated with pegaptanib showed complete regression of neovascularization at week 36, while only 25% of eyes treated with panretinal photocoagulation showed this benefit [71]. Regression of neovascularization associated with PDR after intravitreal pegaptanib has also been reported by other studies [72, 73]. Nevertheless, pegaptanib is not FDA-approved for the treatment of DME or diabetic retinopathy.
Aflibercept. Aflibercept is FDA-approved for the treatment of DME and diabetic retinopathy associated with DME. The DME and VEGF Trap-Eye: Investigation of Clinical Impact (DA VINCI) phase II randomized controlled trial reported that aflibercept was associated with better visual outcomes than focal/grid photocoagulation [74]. Two phase III randomized controlled trials, Study of Intravitreal Administration of VEGF Trap-Eye in Patients with Diabetic Macular Edema (VISTA-DME) and Intravitreal Alfibercept Injection in Vision Impairment Due to DME (VIVID-DME), reported that intravitreal aflibercept was associated with better visual outcomes than focal/grid photocoagulation at 53 weeks [75].
In 2015, the DRCR published the results from protocol T [76]. In this randomized controlled trial, involving 89 participating centers, a total of 660 participants with center-involved DME were randomized to receive aflibercept, bevacizumab, or ranibizumab as frequently as every 4 weeks. Patients would additionally receive focal/grid photocoagulation at or after 6 months if DME persisted or declined. The main outcome measure was mean change in visual acuity at 1 year compared with baseline. Among eyes with baseline visual acuity of 20/40 or better, all three treatment groups showed similar improvements in visual acuity. However, in eyes with baseline visual acuity of 20/50 or worse, 1-year visual acuity improvements in the group treated with aflibercept were significantly better than in both the bevacizumab and ranibizumab groups. There were no statistically significant visual differences between bevacizumab and ranibizumab. Furthermore, aflibercept reduced central subfield thickness on OCT significantly more than both bevacizumab and ranibizumab, while ranibizumab showed significantly better OCT outcomes than bevacizumab.
The protocol T subgroup analysis reported that in eyes with visual acuity of 20/40 or better, aflibercept and ranibizumab showed similar OCT benefits, and they both outperformed bevacizumab. The same trend was observed in eyes with baseline visual acuity of 20/50 or worse. Overall, all three treatments had similar safety profiles. However, post hoc analysis revealed that there were more adverse cardiovascular events in the ranibizumab treatment group. It was concluded that all three anti-VEGF agents substantially improved visual acuity at 1 year, with aflibercept providing significantly better visual acuity results in eyes with poor baseline visual acuity.
2.5 Pars plana vitrectomy
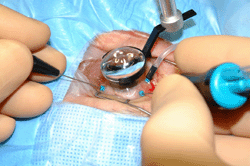
For decades, pars plana vitrectomy has been utilized in the treatment of DME, especially in eyes with concurrent vitreomacular interface abnormalities, but results have been mixed (Figure 4). A meta-analysis of clinical trials evaluated pars plana vitrectomy for DME. While pars plana vitrectomy can provide anatomical and visual acuity improvements at 6 months, functional improvements were minimal when compared with focal/grid photocoagulation. Furthermore, at 12 months, there were minimal overall advantages of pars plana vitrectomy for DME over focal/grid photocoagulation [77]. In a prospective cohort study by the DRCR (protocol D), the effect of pars plana vitrectomy in 87 eyes with DME and vitreomacular traction was evaluated. After 6 months, 68% of eyes had at least a 50% reduction in OCT thickness. Approximately 38% of eyes had a visual improvement of 10 letters or more, but 22% experienced visual impairment of 10 letters or more [78].
 |
 |
Figure 4. Pars plana vitrectomy. Demonstration of pars plana vitrectomy for complications of proliferative diabetic retinopathy (right eye). |
|
Pars plana vitrectomy is generally more beneficial in the treatment of patients with various manifestations of PDR, including non-clearing vitreous hemorrhage and traction retinal detachment (Figure 5). The Diabetic Retinopathy Vitrectomy Study (DRVS) evaluated the benefits of "early" pars plana vitrectomy (within 6 months) in eyes with severe diabetic vitreous hemorrhage. At 2 years follow-up, a significantly higher proportion of vitrectomized eyes had final visual acuity of at least 10/20 than of eyes managed by other treatments [79]. A follow-up study reported similar results after 4 years [80]. In another study, 87% of eyes with non-clearing diabetic vitreous hemorrhage improved by at least 3 lines of visual acuity at 1 year [81].
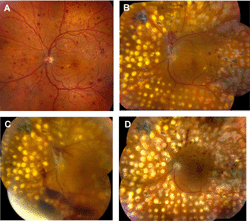 |
 |
Figure 5. Treatment of diabetic retinopathy using panretinal photocoagulation and pars plana vitrectomy. A. Montage fundus photography of left eye, showing early proliferative diabetic retinopathy. B. Montage fundus photography of the same eye several months after treatment with panretinal photocoagulation. There is persistent proliferative diabetic retinopathy. C. Montage fundus photography of the same eye. There is now vitreous hemorrhage (obscuring the view) plus early traction retinal detachment. D. Montage fundus photography of the same eye following pars plana vitrectomy. The vitreous hemorrhage and traction retinal detachment have resolved. |
|
In a randomized controlled trial by the DRCR (protocol N), the effect of intravitreal ranibizumab versus intravitreal saline was compared among 261 eyes with PDR and vitreous hemorrhage. The main outcome measure was the cumulative probability of pars plana vitrectomy after 16 weeks. The cumulative probability of pars plana vitrectomy did not differ significantly between the two groups [82], although a probable biologic effect was seen in improved rates of vitreous hemorrhage clearing and improved visual acuity outcomes in the ranibizumab-treated eyes.
Traction retinal detachment secondary to PDR is another common indication for pars plana vitrectomy (Figure 6). While anatomic success rates of more than 90% have been reported [83, 84], visual outcomes have been mixed. In a representative study, only 50% of eyes with diabetic traction retinal detachment had visual acuity improvement of 3 lines or more after pars plana vitrectomy [81]. Other studies have reported similar results with less than 40% of eyes achieving final visual acuity of at least 20/100 [85, 86].
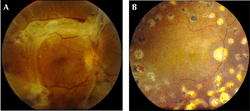 |
 |
Figure 6. Traction retinal detachment. A. Fundus photography of right eye, demonstrating traction retinal detachment. B. Fundus photography of the same eye following pars plana vitrectomy. The traction retinal detachment has resolved. |
|
3. Emerging new treatments for diabetic retinopathy and diabetic macular edema
Both photocoagulation and intravitreal pharmacotherapies (anti-VEGF and corticosteroids) are associated with potentially serious adverse effects. Furthermore, a percentage of patients do not show a favorable response with either of these treatment modalities.
3.1 Proliferative diabetic retinopathy (PDR) and non-proliferative diabetic retinopathy (NPDR)
Inflammatory processes and neurodegeneration have been implicated in the early stages of diabetic retinopathy [87, 88]. It has also been reported that the levels of the neuroprotective substance, somatostatin, were significantly lower in diabetic donor eyes without clinical signs of diabetic retinopathy [89] and in living eyes with PDR or DME compared with non-diabetic eyes [90].
The role of neuroprotective agents in preventing the progression of diabetic retinopathy has been evaluated. Two pilot studies have reported that patients with severe NPDR or early PDR receiving intramuscular administration of somatostatin analogs were less likely to require panretinal photocoagulation [91, 92]. Currently, a European phase II/III study is evaluating the effects of topically administrated neuroprotective substances somatostatin and brimonidine in patients with NPDR [93].
A proof-of-concept randomized controlled trial was set out to evaluate the effects of 50 mg daily doxycycline given for 24 months versus placebo in eyes with severe NPDR and eyes with mild or moderate PDR. The 2-year results showed that foveal sensitivity (measured by photopic visual field) decreased among the placebo group, while it increased in the group receiving doxycycline, when compared with baseline. However, all other anatomical and functional outcomes were not significantly different between the two groups [94].
A phase II randomized controlled trial evaluated the effects of oral therapy with a glycosaminoglycan (GAG), sulodexide (Vessel Due F, Aju Pharm, Seoul, South Korea, under license from Alfa Wassermann, Bologna, Italy), on hard exudates in patients with NPDR, and reported a significant reduction in these exudates compared with placebo [95]. The premise for this clinical trial was that in diabetic retinopathy, similar to diabetic nephropathy, GAGs are replaced by collagen in the retinal capillary basement membrane, leading to changes in the permeability of these capillaries [95, 96]. Currently, the role of dietary supplements such as alpha-lipoic acid and multi-component nutritional capsules in the management of NPDR are also being evaluated in a number of clinical trials [97, 98].
3.2 Diabetic macular edema (DME)
Novel agents are being evaluated in the treatment of DME. Angiopoietin 2 is a protein that acts as an antagonist of the tunica internal endothelial cell kinase 2 (TIE2) receptor, which promotes increased vascular permeability and angiogenesis, and which has been reported to be increased in eyes with DME. A novel substance, AKB-9778 (Aerpio Therapeutics Inc., Cincinnati, OH), has been developed to activate TIE2 with the aim of reducing vascular permeability [99]. This phase II randomized controlled trial included patients with DME receiving subcutaneous injections (5 mg, 15 mg, 22.5 mg, or 30 mg) of AKB-9778 twice a day for 4 weeks. The drug was well tolerated with no safety concerns, and showed improvements in visual acuity and reduction of OCT thickness in approximately 40% of patients receiving 15 mg or more [100].
Among other chemokines, CCL-2 (chemokine ligand 2) is a proinflammatory ligand. Its levels are significantly increased in rats with diabetic retinopathy. CCL-2 knockout diabetic rats had significantly less retinal vascular leakage [101]. PF-04634817 (Pfizer, New York, NY, USA), an antagonist of two chemokine receptors CCR2 and CCR5, is currently being evaluated in a phase II randomized controlled trial for the treatment of DME [102].
Components of the plasma kallikrein-kinin system are increased in the vitreous fluid of patients with advanced DR [103]. Activation of this system has been reported to increase retinal vascular leakage in rats [104]. A recently initiated phase I study aims to evaluate intravitreal injection of a plasma kallikrein-inhibiting agent, KVD001, in patients with DME [105].
Small interfering RNAs (siRNAs) can be designed to bind to their target genes and prevent their expression. One such siRNA, bevasiranib (Cand5, Opko Health Inc., Miami, FL, USA), was made to inhibit the expression of VEGF. Preliminary results from the RNA interference (RNAi) Assessment of Bevasiranib in DME (RACE) study, evaluating three different doses of this novel substance, showed a reduction of OCT thickness [106]. However, the official results from the phase II study have not yet been published [107].
Another siRNA, PF-04523655 (Quark, Fremont, CA, USA and/ Pfizer, New York, NY, USA), aims to inhibit the expression of the RTP801 gene, which is upregulated during hypoxia. The Dose-Ranging Evaluation of Intravitreal siRNA PF-04523655 for DME (DEGAS) study evaluated the safety and efficacy of this novel drug (as an intravitreal injection) versus focal/grid photocoagulation. Preliminary results showed improved visual acuity in all treatment groups [108]. Currently, a new dose-escalation phase II randomized controlled trial is underway evaluating the dosing and efficacy of PF-04523655 alone and in combination with ranibizumab versus ranibizumab alone [109].
Sirolimus, also known as rapamycin, has both antifungal and immunosuppressive properties, and leads to inhibition of the mammalian target of rapamycin (mTOR), which is involved in angiogenic pathways [110]. A phase I/II study involving subconjunctival sirolimus in patients with DME reported no safety concerns at 1 year. However, efficacy was not adequately demonstrated because of the small number of participants (n = 5) and the non-randomized nature of the study [111].
Luminate (formerly ALG-1001, Allegro Ophthalmics, LLC, San Juan Capistrano, CA) is an engineered oligopeptide that targets integrin receptors involved in retinal angiogenesis. The preliminary safety and efficacy data from intravitreal injection of this drug in patients with DME showed that about 50% of the participants had visual improvements of 3 or more letters after 3 monthly injections, and among these patients, there was an 83% reduction in OCT thickness. No patients had worsening of visual acuity or worsening of OCT thickness over 5 months [112]. A phase II randomized controlled trial was initiated, evaluating 3 different doses of luminate versus bevacizumab or photocoagulation [113]. Other integrin inhibiting agents such as ATN-161 (an integrinα5β1 inhibitor) [114] and an anti-integrin platelet aggregation inhibitor (PAI) protein-derived snake venom [115] have been reported to have anti-angiogenic effects in animal models.
4. Novel drug delivery methods
Novel drug delivery methods to the posterior segment of the eye could prove promising for the treatment of diabetic retinopathy. Encapsulated cell technology (ECT) allows a genetically modified group of cell lines expressing the gene of interest to be encapsulated in synthetic semi-permeable capsules, which allows diffusion of nutrients to these cells while protecting them from the host’s defense mechanisms [116]. These capsules can be surgically implanted in target areas including the posterior segment of the eye. This technology has already been tested in a number of neurodegenerative diseases. Proof-of-concept and phase I/II studies are currently underway to evaluate the efficacy of capsules with anti-VEGF activity [117].
Another treatment strategy under investigation is the surgically implanted small intraocular pump that releases a predetermined amount of drugs at scheduled intervals. One potential advantage of these implantable reservoir pumps is that they can be easily refilled without the need to remove or insert a whole new pump apparatus. One such example of this technology is the Replenish MicroPump (Replenish, Pasadena, California, USA). This small refillable pump is implanted in a similar way as a glaucoma drainage device (placed in the subconjunctival space), with its cannula extending into the vitreous. Initially, the implant was successfully tested in dogs [118]. The feasibility and safety of this implant filled with ranibizumab was evaluated in a prospective non-randomized study using human participants with DME [119]. No serious adverse effects were reported at 3 months.
Another newly developed reservoir implant is the Port Shunt Delivery (ForSight; Menlo Park, CA, USA). This is also a refillable drug delivery system, which is currently being investigated in age-related macular degeneration [120].
5. Novel utilizations of diagnosis and testing modalities in diabetic retinopathy
Advances in imaging modalities such as fundus photography, fluorescein angiography, OCT, and other testing tools provide valuable means of evaluating, characterizing, and managing different stages of diabetic retinopathy. A prospective study of 109 diabetic eyes without PDR at baseline included patients with at least 1 predominantly peripheral diabetic retinopathy lesion (defined as a lesion of which 50% lies outside of the ETDRS 7 standard photographic fields). The lesions were identified using 200° ultrawide field imaging. The patients were 3.2 times more likely to have a ≥2-step diabetic retinopathy progression and 4.7 times more likely to develop PDR when compared with eyes without predominantly peripheral lesions [121]. The investigators concluded that these peripheral lesions could provide additional important information on the risk of diabetic retinopathy progression.
In a cross-sectional study, 45 patients (15 with PDR treated with PRP, 15 with untreated PDR, and 15 age-matched controls) underwent multiple testing modalities, including contrast sensitivity, frequency doubling perimetry, Humphrey visual fields, photo-stress recovery, and dark adaptation. Compared with controls, both PRP-treated PDR patients and untreated PDR patients had significant changes in retinal structure and function using multiple modalities, with untreated patients exhibiting more changes in inner and PRP-treated patients in outer retinal structure [122].
6. Conclusions
Diabetic retinopathy, including DME and PDR, remains one of the most critical causes of visual loss worldwide. For decades, there were only surgical treatment options, using either photocoagulation or pars plana vitrectomy. More recently, pharmacological therapies have become more widely accepted. In particular, intravitreal pharmacotherapies have become popular in the management of DME. Although PRP is still standard treatment for PDR, the recent FDA approvals of ranibizumab and aflibercept for patients with diabetic retinopathy associated with DME, and the promising results of these agents in diabetic retinopathy suggest a potential role in pharmacologic treatment of PDR. The recent DRCR finding (protocol S) that ranibizumab was non-inferior to PRP in patients with PDR supports a role for pharmacologic treatment of proliferative eye disease.
Currently, intravitreal anti-VEGF agents are frequently used as first-line therapies in the management of center-involved DME. Focal/grid photocoagulation remains beneficial for patients with non-center-involved DME, and may reduce the burden of frequent intravitreal injections. Intravitreal corticosteroids do also have a role in the management of DME, but they are frequently used as second-line agents. Ongoing clinical trials and other investigations will determine the role of novel agents in the treatment of diabetic retinopathy.
Disclosures: Dr. Schwartz has received consulting fees from Alimera and Bausch + Lomb and writers' fees from Vindico. All other authors have no conflict of interests to disclose.
Acknowledgments:
This work was partially supported by NIH Center Core Grant P30EY014801 and an unrestricted grant from Research to Prevent Blindness, New York, NY.
References
- Centers for Disease Control and Prevention. National diabetes statistics report, 2014. http://www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf, accessed May 6, 2015. [DOD]
- Zhang X, Saaddine JB, Chou CF, Cotch MF, Cheng YJ, Geiss LS, Gregg EW, Albright AL, Klein BE, Klein R. Prevalence of diabetic retinopathy in the United States, 2005-2008. JAMA 2010. 304(6):649-656. [DOD] [CrossRef]
- Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, Chen SJ, Dekker JM, Fletcher A, Grauslund J, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012. 35(3):556-564. [DOD] [CrossRef]
- Sivaprasad S, Gupta B, Gulliford MC, Dodhia H, Mohamed M, Nagi D, Evans JR. Ethnic variations in the prevalence of diabetic retinopathy in people with diabetes attending screening in the United Kingdom (DRIVE UK). Plos One 2012. 7(3):e32182. [DOD] [CrossRef]
- Wong TY, Klein R, Islam FM, Cotch MF, Folsom AR, Klein BE, Sharrett AR, Shea S. Diabetic retinopathy in a multi-ethnic cohort in the United States. Am J Ophthalmol 2006. 141(3):446-455. [DOD] [CrossRef]
- Sivaprasad S, Gupta B, Crosby-Nwaobi R, Evans J. Prevalence of diabetic retinopathy in various ethnic groups: a worldwide perspective. Surv Ophthalmol 2012. 57(4):347-370. [DOD] [CrossRef]
- Thomas RL, Dunstan FD, Luzio SD, Chowdhury SR, North RV, Hale SL, Gibbins RL, Owens DR. Prevalence of diabetic retinopathy within a national diabetic retinopathy screening service. Br J Ophthalmol 2015. 99(1):64-68. [DOD] [CrossRef]
- Varma R, Bressler NM, Doan QV, Gleeson M, Danese M, Bower JK, Selvin E, Dolan C, Fine J, Colman S, et al. Prevalence of and risk factors for diabetic macular edema in the United States. JAMA Ophthalmol 2014. 132(11):1334-1340. [DOD] [CrossRef]
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998. 352(9131):837-853. [DOD] [CrossRef]
- UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998. 352(9131):854-865. [DOD] [CrossRef]
- UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998. 317(7160):703-713. [DOD] [CrossRef]
- The Diabetes and Complications Trial Research Group. The Effect of Intensive Treatment of Diabetes on the Development and Progression of Long-Term Complications in Insulin-Dependent Diabetes Mellitus. N Engl J Med 1993. 329(14):977-986. [DOD] [CrossRef]
- Dirani M, Xie J, Fenwick E, Benarous R, Rees G, Wong TY, Lamoureux EL. Are obesity and anthropometry risk factors for diabetic retinopathy? The diabetes management project. Invest Ophthalmol Vis Sci 2011. 52(7):4416-4421. [DOD] [CrossRef]
- Chaturvedi N, Sjoelie AK, Porta M, Aldington SJ, Fuller JH, Songini M, Kohner EM, EURODIAB Prospective Complications Study. Markers of insulin resistance are strong risk factors for retinopathy incidence in type 1 diabetes. Diabetes Care 2001. 24(2):284-289. [DOD] [CrossRef]
- Henricsson M, Nyström L, Blohme G, Ostman J, Kullberg C, Svensson M, Scholin A, Arnqvist HJ, Bjork E, Bolinder J, et al. The incidence of retinopathy 10 years after diagnosis in young adult people with diabetes: results from the nationwide population-based Diabetes Incidence Study in Sweden (DISS). Diabetes Care 2003. 26(2):349-354. [DOD] [CrossRef]
- Rema M, Srivastava BK, Anitha B, Deepa R, Mohan V. Association of serum lipids with diabetic retinopathy in urban South Indians - the Chennai Urban Rural Epidemiology Study (CURES) Eye Study 2. Diabet Med J Br Diabet Assoc 2006. 23(9):1029-1036. [DOD] [CrossRef]
- Sen D, Ghosh S, Roy D. Correlation of C-reactive protein and body mass index with diabetic retinopathy in Indian population. Diabetes Metab Syndr 2015. 9(1):28-29. [DOD] [CrossRef]
- Wong TY, Cheung N, Tay WT, Wang JJ, Aung T, Saw SM, Lim SC, Tai ES, Mitchell P. Prevalence and risk factors for diabetic retinopathy: the Singapore Malay Eye Study. Ophthalmology 2008. 115(11):1869-1875. [DOD] [CrossRef]
- Lim LS, Tai ES, Mitchell P, Wang JJ, Tay WT, Lamoureux E, Wong TY. C-reactive protein, body mass index, and diabetic retinopathy. Invest Ophthalmol Vis Sci 2010. 51(9):4458-4463. [DOD] [CrossRef]
- Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract 2014. 103(2):137-149. [DOD] [CrossRef]
- Saaddine JB, Honeycutt AA, Narayan KM, Zhang X, Klein R, Boyle JP. Projection of diabetic retinopathy and other major eye diseases among people with diabetes mellitus: United States, 2005-2050. Arch Ophthalmol 2008. 126(12):1740-1747. [DOD] [CrossRef]
- Jones CD, Greenwood RH, Misra A, Bachmann MO. Incidence and progression of diabetic retinopathy during 17 years of a population-based screening program in England. Diabetes Care 2012. 35(3):592-596. [DOD] [CrossRef]
- Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Research Group, Lachin JM, White NH, Hainsworth DP, Sun W, Cleary PA, Nathan DM. Effect of intensive diabetes therapy on the progression of diabetic retinopathy in patients with type 1 diabetes: 18 years of follow-up in the DCCT/EDIC. Diabetes 2015. 64(2):631-642. [DOD] [CrossRef]
- Do DV, Wang X, Vedula SS, Marrone M, Sleilati G, Hawkins BS, Frank RN. Blood pressure control for diabetic retinopathy. Cochrane Database Syst Rev 2015. 1:CD006127. [DOD]
- Chew EY, Davis MD, Danis RP, Lovato JF, Perdue LH, Greven C, Genuth S, Goff DC, Leiter LA, Ismail-Beigi F, et al. The effects of medical management on the progression of diabetic retinopathy in persons with type 2 diabetes: the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Eye Study. Ophthalmology 2014. 121(12):2443-2451. [DOD] [CrossRef]
- Noonan JE, Jenkins AJ, Ma JX, Keech AC, Wang JJ, Lamoureux EL. An update on the molecular actions of fenofibrate and its clinical effects on diabetic retinopathy and other microvascular end points in patients with diabetes. Diabetes 2013. 62(12):3968-3975. [DOD] [CrossRef]
- Keech AC, Mitchell P, Summanen PA, O'Day J, Davis TM, Moffitt MS, Taskinen MR, Simes RJ, Tse D, Williamson E, et al. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet 2007. 370(9600):1687-1697. [DOD] [CrossRef]
- The Diabetic Retinopathy Study Research Group. Indications for photocoagulation treatment of diabetic retinopathy: diabetic retinopathy study report no. 14. Int Ophthalmol Clin 1987. 27(4):239-253. [DOD] [CrossRef]
- The Early Treatment Diabetic Retinopathy Study (ETDRS). Early photocoagulation for diabetic retinopathy. ETDRS report number 9. Ophthalmology 1991. 98(5 Suppl):766-785. [DOD]
- Evans JR, Michelessi M, Virgili G. Laser photocoagulation for proliferative diabetic retinopathy. Cochrane Database Syst Rev 2014. 11:CD011234. [DOD]
- Early Treatment Diabetic Retinopathy Study Research Group (ETDRS). Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Arch Ophthalmol 1985. 103(12):1796-1806. [DOD] [CrossRef]
- Tolentino M. Systemic and ocular safety of intravitreal anti-VEGF therapies for ocular neovascular disease. Surv Ophthalmol 2011. 56(2):95-113. [DOD] [CrossRef]
- Sampat KM, Garg SJ. Complications of intravitreal injections. Curr Opin Ophthalmol 2010. 21(3):178-183. [DOD] [CrossRef]
- Diabetic Retinopathy Clinical Research Network. A Randomized Trial Comparing Intravitreal Triamcinolone Acetonide and Focal/Grid Photocoagulation for Diabetic Macular Edema. Ophthalmology 2008. 115(9):1447-1449. [DOD] [CrossRef]
- Diabetic Retinopathy Clinical Research Network, Elman MJ, Aiello LP, Beck RW, Bressler NM, Bressler SB, Edwards AR, Ferris FL 3rd, Friedman SM, Glassman AR, et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology 2010. 117(6):1064-1077. [DOD] [CrossRef]
- Maia OO Jr, Takahashi BS, Costa RA, Scott IU, Takahashi WY. Combined laser and intravitreal triamcinolone for proliferative diabetic retinopathy and macular edema: one-year results of a randomized clinical trial. Am J Ophthalmol 2009. 147(2):291-297. [DOD] [CrossRef]
- Zacks DN, Johnson MW. Combined intravitreal injection of triamcinolone acetonide and panretinal photocoagulation for concomitant diabetic macular edema and proliferative diabetic retinopathy. Retina 2005. 25(2):135-140. [DOD] [CrossRef]
- Zein WM, Baha'n N, Jurdi FA, Schakal A, Bashshur ZF. Panretinal photocoagulation and intravitreal triamcinolone acetonide for the management of proliferative diabetic retinopathy with macular edema. Retina 2006. 26(2):137-142. [DOD] [CrossRef]
- Bressler SB, Qin H, Melia M, Bressler NM, Beck RW, Chan CK, Grover S, Miller DG, Diabetic Retinopathy Clinical Research Network. Exploratory analysis of the effect of intravitreal ranibizumab or triamcinolone on worsening of diabetic retinopathy in a randomized clinical trial. JAMA Ophthalmol 2013. 131(8):1033-1040. [DOD] [CrossRef]
- Callanan DG, Gupta S, Boyer DS, Ciulla TA, Singer MA, Kuppermann BD, Liu CC, Li XY, Hollander DA, Schiffman RM, et al. Dexamethasone intravitreal implant in combination with laser photocoagulation for the treatment of diffuse diabetic macular edema. Ophthalmology 2013. 120(9):1843-1851. [DOD] [CrossRef]
- Boyer DS, Yoon YH, Belfort R, Bandello F, Maturi RK, Augustin AJ, Li XY, Cui H, Hashad Y, Whitcup SM, et al. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology 2014. 121(10):1904-1914. [DOD] [CrossRef]
- Pearson PA, Comstock TL, Ip M, Callanan D, Morse LS, Ashton P, Levy B, Mann ES, Eliott D. Fluocinolone acetonide intravitreal implant for diabetic macular edema: a 3-year multicenter, randomized, controlled clinical trial. Ophthalmology 2011. 118(8):1580-1587. [DOD] [CrossRef]
- Campochiaro PA, Brown DM, Pearson A, Ciulla T, Boyer D, Holz FG, Tolentino M, Gupta A, Duarte L, Madreperla S, et al. Long-term benefit of sustained-delivery fluocinolone acetonide vitreous inserts for diabetic macular edema. Ophthalmology 2011. 118(4):626-635. [DOD] [CrossRef]
- Campochiaro PA, Brown DM, Pearson A, Chen S, Boyer D, Ruiz-Moreno J, Garretson B, Gupta A, Hariprasad SM, Bailey C, et al. Sustained delivery fluocinolone acetonide vitreous inserts provide benefit for at least 3 years in patients with diabetic macular edema. Ophthalmology 2012. 119(10):2125-2132. [DOD] [CrossRef]
- Adamis AP, Miller JW, Bernal MT, D'Amico DJ, Folkman J, Yeo TK, Yeo KT. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol 1994. 118(4):445-450. [DOD] [CrossRef]
- Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, Pasquale LR, Thieme H, Iwamoto MA, Park JE, et al. Vascular Endothelial Growth Factor in Ocular Fluid of Patients with Diabetic Retinopathy and Other Retinal Disorders. N Engl J Med 1994. 331(22):1480-1487. [DOD] [CrossRef]
- Diabetic Retinopathy Clinical Research Network, Scott IU, Edwards AR, Beck RW, Bressler NM, Chan CK, Elman MJ, Friedman SM, Greven CM, Maturi RK, et al. A phase II randomized clinical trial of intravitreal bevacizumab for diabetic macular edema. Ophthalmology 2007. 114(10):1860-1867. [DOD] [CrossRef]
- Rajendram R, Fraser-Bell S, Kaines A, Michaelides M, Hamilton RD, Esposti SD, Peto T, Egan C, Bunce C, Leslie RD, et al. A 2-year prospective randomized controlled trial of intravitreal bevacizumab or laser therapy (BOLT) in the management of diabetic macular edema: 24-month data: report 3. Arch Ophthalmol 2012. 130(8):972-979. [DOD] [CrossRef]
- Soheilian M, Ramezani A, Obudi A, Bijanzadeh B, Salehipour M, Yaseri M, Ahmadieh H, Dehghan MH, Azarmina M, Moradian S, et al. Randomized trial of intravitreal bevacizumab alone or combined with triamcinolone versus macular photocoagulation in diabetic macular edema. Ophthalmology 2009. 116(6):1142-1150. [DOD] [CrossRef]
- Avery RL, Pearlman J, Pieramici DJ, Rabena MD, Castellarin AA, Nasir MA, Giust MJ, Wendel R, Patel A. Intravitreal bevacizumab (Avastin) in the treatment of proliferative diabetic retinopathy. Ophthalmology. 2006. 113(10):1695. [DOD]
- Spaide RF, Fisher YL. Intravitreal bevacizumab (Avastin) treatment of proliferative diabetic retinopathy complicated by vitreous hemorrhage. Retina 2006. 26(3):275-278. [DOD] [CrossRef]
- Arevalo JF, Wu L, Sanchez JG, Maia M, Saravia MJ, Fernandez CF, Evans T. Intravitreal bevacizumab (Avastin) for proliferative diabetic retinopathy: 6-months follow-up. Eye 2009. 23(1):117-123. [DOD] [CrossRef]
- Jorge R, Costa RA, Calucci D, Cintra LP, Scott IU. Intravitreal bevacizumab (Avastin) for persistent new vessels in diabetic retinopathy (IBEPE study). Retina 2006. 26(9):1006-1013. [DOD] [CrossRef]
- Mirshahi A, Roohipoor R, Lashay A, Mohammadi SF, Abdoallahi A, Faghihi H. Bevacizumab-augmented retinal laser photocoagulation in proliferative diabetic retinopathy: a randomized double-masked clinical trial. Eur J Ophthalmol 2008. 18(2):263-269. [DOD]
- Tonello M, Costa RA, Almeida FPP, Barbosa JC, Scott IU, Jorge R. Panretinal photocoagulation versus PRP plus intravitreal bevacizumab for high-risk proliferative diabetic retinopathy (IBeHi study). Acta Ophthalmol 2008. 86(4):385-389. [DOD] [CrossRef]
- Rizzo S, Genovesi-Ebert F, Di Bartolo E, Vento A, Miniaci S, Williams G. Injection of intravitreal bevacizumab (Avastin) as a preoperative adjunct before vitrectomy surgery in the treatment of severe proliferative diabetic retinopathy (PDR). Graefes Arch Clin Exp Ophthalmol 2008. 246(6):837-842. [DOD] [CrossRef]
- Zhao LQ, Zhu H, Zhao PQ, Hu YQ. A systematic review and meta-analysis of clinical outcomes of vitrectomy with or without intravitreal bevacizumab pretreatment for severe diabetic retinopathy. Br J Ophthalmol 2011. 95(9):1216-1222. [DOD] [CrossRef]
- Chen E, Park CH. Use of intravitreal bevacizumab as a preoperative adjunct for tractional retinal detachment repair in severe proliferative diabetic retinopathy. Retina 2006. 26(6):699-700. [DOD] [CrossRef]
- Nguyen QD, Brown DM, Marcus DM, Boyer DS, Patel S, Feiner L, Gibson A, Sy J, Rundle AC, Hopkins JJ, et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology 2012. 119(4):789-801. [DOD] [CrossRef]
- Mitchell P, Bandello F, Schmidt-Erfurth U, Lang GE, Massin P, Schlingermann RO, Sutter F, Simader C, Burian G, Gerstner O, et al. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology 2011. 118(4):615-625. [DOD] [CrossRef]
- Lang GE, Berta A, Eldem BM, Simader C, Sharp D, Holz FG, Sutter F, Gerstner O, Mitchell P, RESTORE Extension Study Group. Two-year safety and efficacy of ranibizumab 0.5 mg in diabetic macular edema: interim analysis of the RESTORE extension study. Ophthalmology 2013. 120(10):2004-2012. [DOD] [CrossRef]
- Massin P, Bandello F, Garweg JG, Hansen LL, Harding SP, Larsen M, Mitchell P, Sharp D, Wolf-Schnurrbusch UE, Gekkieva M, et al. Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE Study): a 12-month, randomized, controlled, double-masked, multicenter phase II study. Diabetes Care 2010. 33(11):2399-2405. [DOD] [CrossRef]
- Nguyen QD, Shah SM, Heier JS, Do DV, Lim J, Boyer D, Abraham P, Campochiaro PA, READ-2 Study Group. Primary End Point (Six Months) Results of the Ranibizumab for Edema of the mAcula in diabetes (READ-2) study. Ophthalmology 2009. 116(11):2175-2181. [DOD] [CrossRef]
- Nguyen QD, Shah SM, Khwaja AA, Channa R, Hatef E, Do DV, Boyer D, Heier JS, Abraham P, Thach AB, et al. Two-year outcomes of the ranibizumab for edema of the mAcula in diabetes (READ-2) study. Ophthalmology 2010. 117(11):2146-2151. [DOD] [CrossRef]
- Elman MJ, Bressler NM, Qin H, Beck RW, Ferris FL 3rd, Friedman SM, Glassman AR, Scott IU, Stockdale CR, Sun JK, et al. Expanded 2-year follow-up of ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology 2011. 118(4):609-614. [DOD] [CrossRef]
- Diabetic Retinopathy Clinical Research Network Writing Committee, Aiello LP, Beck RW, Bressler NM, Browning DJ, Chalam KV, Davis M, Ferris FL 3rd, Glassman AR, Maturi RK, et al. Rationale for the diabetic retinopathy clinical research network treatment protocol for center-involved diabetic macular edema. Ophthalmology 2011. 118(12):e5-e14. [DOD] [CrossRef]
- Elman MJ, Ayala A, Bressler NM, Browning D, Flaxel CJ, Glassman AR, Jampol LM, Stone TW, Diabetic Retinopathy Clinical Research Network. Intravitreal ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: 5-year randomized trial results. Ophthalmology 2015. 122(2):375-381. [DOD] [CrossRef]
- Brown DM, Nguyen QD, Marcus DM, Boyer DS, Patel S, Feiner L, Schlottmann PG, Rundle AC, Zhang J, Rubio RG, et al. Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology 2013. 120(10):2013-2022. [DOD] [CrossRef]
- Writing Committee for the Diabetic Retinopathy Clinical Research Network, Gross JG, Glassman AR, Jampol LM, Inusah S, Aiello LP, Antoszyk AN, Baker CW, Berger BB, Bressler NM, Browning D, Elman MJ, Ferris FL 3rd, Friedman SM, Marcus DM, Melia M, Stockdale CR, Sun JK, Beck RW. Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA 2015. 314(20):2137-2146. [DOD] [CrossRef]
- Sultan MB, Zhou D, Loftus J, Dombi T, Ice KS, Macugen 1013 Study Group. A phase 2/3, multicenter, randomized, double-masked, 2-year trial of pegaptanib sodium for the treatment of diabetic macular edema. Ophthalmology 2011. 118(6):1107-1118. [DOD] [CrossRef]
- Gonzalez VH, Giuliari GP, Banda RM, Guel DA. Intravitreal injection of pegaptanib sodium for proliferative diabetic retinopathy. Br J Ophthalmol 2009. 93(11):1474-1478. [DOD] [CrossRef]
- Mendrinos E, Donati G, Pournaras CJ. Rapid and persistent regression of severe new vessels on the disc in proliferative diabetic retinopathy after a single intravitreal injection of pegaptanib. Acta Ophthalmol 2009. 87(6):683-684. [DOD] [CrossRef]
- Krzystolik MG, Filippopoulos T, Ducharme JF, Loewenstein JI. Pegaptanib as an adjunctive treatment for complicated neovascular diabetic retinopathy. Arch Ophthalmol 2006. 124(6):920-921. [DOD] [CrossRef]
- Do DV, Nguyen QD, Boyer D, Schmidt-Erfurth U, Brown DM, Vitti R, Berliner AJ, Gao B, Zeitz O, Ruckert R, et al. One-year outcomes of the da Vinci Study of VEGF Trap-Eye in eyes with diabetic macular edema. Ophthalmology 2012. 119(8):1658-1665. [DOD] [CrossRef]
- Korobelnik JF, Do DV, Schmidt-Erfurth U, Boyer DS, Holz FG, Heier JS, Midena E, Kaiser PK, Terasaki H, Marcus DM, et al. Intravitreal aflibercept for diabetic macular edema. Ophthalmology 2014. 121(11):2247-2254. [DOD] [CrossRef]
- Diabetic Retinopathy Clinical Research Network. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med 2015. 372(13):1193-1203. [DOD] [CrossRef]
- Simunovic MP, Hunyor AP, Ho IV. Vitrectomy for diabetic macular edema: a systematic review and meta-analysis. Can J Ophthalmol 2014. 49(2):188-195. [DOD] [CrossRef]
- Diabetic Retinopathy Clinical Research Network. Vitrectomy outcomes in eyes with diabetic macular edema and vitreomacular traction. Ophthalmology 2010. 117(6):1087-1093. [DOD] [CrossRef]
- The Diabetic Retinopathy Vitrectomy Study Research Group. Early vitrectomy for severe vitreous hemorrhage in diabetic retinopathy. Two-year results of a randomized trial. Diabetic Retinopathy Vitrectomy Study report 2. Arch Ophthalmol 1985. 103(11):1644-1652. [DOD] [CrossRef]
- The Diabetic Retinopathy Vitrectomy Study Research Group. Early vitrectomy for severe vitreous hemorrhage in diabetic retinopathy. Four-year results of a randomized trial: Diabetic Retinopathy Vitrectomy Study Report 5. Arch Ophthalmol 1990. 108(7):958-964. [DOD] [CrossRef]
- Gupta B, Sivaprasad S, Wong R, Laidlaw A, Jackson TL, McHugh D, Williamson TH. Visual and anatomical outcomes following vitrectomy for complications of diabetic retinopathy: The DRIVE UK Study. Eye 2012. 26(4):510-516. [DOD] [CrossRef]
- Diabetic Retinopathy Clinical Research Network. Randomized clinical trial evaluating intravitreal ranibizumab or saline for vitreous hemorrhage from proliferative diabetic retinopathy. JAMA Ophthalmol 2013. 131(3):283-293. [DOD] [CrossRef]
- Oshima Y, Shima C, Wakabayashi T, Kusaka S, Shiraga F, Ohji M, Tano Y. Microincision vitrectomy surgery and intravitreal bevacizumab as a surgical adjunct to treat diabetic traction retinal detachment. Ophthalmology 2009. 116(5):927-938. [DOD] [CrossRef]
- Tao Y, Jiang YR, Li XX, Gao L, Jonas JB. Long-term results of vitrectomy without endotamponade in proliferative diabetic retinopathy with tractional retinal detachment. Retina 2010. 30(3):447-451. [DOD] [CrossRef]
- Mason JO 3rd, Colagross CT, Haleman T, Fuller JJ, White MF, Feist RM, Emond TL, McGwin G Jr. Visual outcome and risk factors for light perception and no light perception vision after vitrectomy for diabetic retinopathy. Am J Ophthalmol 2005. 140(2):231-235. [DOD]
- Yorston D, Wickham L, Benson S, Bunce C, Sheard R, Charteris D. Predictive clinical features and outcomes of vitrectomy for proliferative diabetic retinopathy. Br J Ophthalmol 2008. 92(3):365-368. [DOD] [CrossRef]
- Kern TS. Contributions of inflammatory processes to the development of the early stages of diabetic retinopathy. Exp Diabetes Res 2007. 2007:95103. [DOD]
- Barber AJ. A new view of diabetic retinopathy: a neurodegenerative disease of the eye. Prog Neuropsychopharmacol Biol Psychiatry 2003. 27(2):283-290. [DOD] [CrossRef]
- Carrasco E, Hernandez C, Miralles A, Huguet P, Farres J, Simo R. Lower somatostatin expression is an early event in diabetic retinopathy and is associated with retinal neurodegeneration. Diabetes Care 2007. 30(11):2902-2908. [DOD] [CrossRef]
- Simo R, Carrasco E, Fonollosa A, Garcia-Arumi J, Casamitjana R, Hernandez C. Deficit of somatostatin in the vitreous fluid of patients with diabetic macular edema. Diabetes Care 2007. 30(3):725-727. [DOD] [CrossRef]
- Grant MB, Mames RN, Fitzgerald C, Hazariwala KM, Cooper-DeHoff R, Caballero S, Estes KS. The efficacy of octreotide in the therapy of severe nonproliferative and early proliferative diabetic retinopathy: a randomized controlled study. Diabetes Care 2000. 23(4):504-509. [DOD] [CrossRef]
- Boehm BO, Lang GK, Jehle PM, Feldman B, Lang GE. Octreotide reduces vitreous hemorrhage and loss of visual acuity risk in patients with high-risk proliferative diabetic retinopathy. Horm Metab Res 2001. 33(5):300-306. [DOD] [CrossRef]
- Trial to Assess the Efficacy of Neuroprotective Drugs Administered Topically to Prevent or Arrest Diabetic Retinopathy (EUROCONDOR). https://clinicaltrials.gov/ct/show/NCT01726075, accessed March 7, 2015. [DOD]
- Scott IU, Jackson GR, Quillen DA, Larsen M, Klein R, Liao J, Holfort S, Munch IC, Gardner TW. Effect of doxycycline vs placebo on retinal function and diabetic retinopathy progression in patients with severe nonproliferative or non-high-risk proliferative diabetic retinopathy: a randomized clinical trial. JAMA Ophthalmol 2014. 132(5):535-543. [DOD] [CrossRef]
- Song JH, Chin HS, Kwon OW, Lim SJ, Kim HK, for the DRESS Research Group. Effect of sulodexide in patients with non-proliferative diabetic retinopathy: diabetic retinopathy sulodexide study (DRESS). Graefes Arch Clin Exp 2014. In press. [DOD]
- Gambaro G, Skrha J, Ceriello A. Glycosaminoglycan therapy for long-term diabetic complications? Diabetologia 1998. 41(8):975-979. [DOD]
- Diabetes Visual Function Supplement Study (DiVFuSS). https://clinicaltrials.gov/ct/show/NCT01646047, accessed May 7, 2015. [DOD]
- The Use of Alpha Lipoic Acid for the Treatment and Prevention of Diabetic Retinopathy (ALA-TPD). https://clinicaltrials.gov/ct2/show/NCT01880372, accessed May 7, 2015. [DOD]
- Shen J, Frye M, Lee BL, Reinardy JL, McClung JM, Ding K, Kojima M, Xia H, Seidel C, Lima e Silva R, et al. Targeting VE-PTP activates TIE2 and stabilizes the ocular vasculature. J Clin Invest 2014. 124(10):4564-4576. [DOD] [CrossRef]
- Campochiaro PA, Sophie R, Tolentino M, Miller DM, Browning D, Boyer DS, Heier JS, Gambino L, Withers B, Brigell M, et al. Treatment of diabetic macular edema with an inhibitor of vascular endothelial-protein tyrosine phosphatase that activates tie2. Ophthalmology 2015. 122(3):545-554. [DOD] [CrossRef]
- Rangasamy S, McGuire PG, Franco Nitta C, Monickaraj F, Oruganti SR, Das A. Chemokine-mediated monocyte trafficking into the retina: role of inflammation in alteration of the blood-retinal barrier in diabetic retinopathy. Plos One 2014. 9(10):e108508. [DOD] [CrossRef]
- A phase 2, multi-center study to compare the efficacy and safety of a chemokine CCR2/5 receptor antagonist with ranibizumab in adults with diabetic macular edema. https://www.clinicaltrials.gov/ct2/show/NCT01994291, accessed May 7, 2015. [DOD]
- Gao BB, Chen X, Timothy N, Aiello LP, Feener EP. Characterization of the vitreous proteome in diabetes without diabetic retinopathy and diabetes with proliferative diabetic retinopathy. J Proteome Res 2008. 7(6):2516-2525. [DOD] [CrossRef]
- Liu J, Feener EP. Plasma kallikrein-kinin system and diabetic retinopathy. Biol Chem 2013. 394(3):319-328. [DOD]
- A phase I single ascending dose study of the intravitreal plasma kallikrein inhibitor KVD001 in subjects with DME. https://www.clinicaltrials.gov/ct2/show/NCT01994291, accessed May 7, 2015. [DOD]
- Singerman LJ. Intravitreal bevasiranib in exudative age-related macular degeneration or diabetic macular edema. 25th Annual Meeting of the American Society of Retina Specialists, December 1-5 2007, Indian Wells, CA. [DOD]
- Safety and Efficacy Study of Small Interfering RNA Molecule (Cand5) to Treat Diabetic Macular Edema. https://clinicaltrials.gov/ct2/show/NCT00306904, accessed May 7, 2015. [DOD]
- Nguyen QD, Schachar RA, Nduaka CI, Sperling M, Basile AS, Klamerus KJ, Chi-Burris K, Yan E, Paggiarino DA, Rosenblatt I, et al. Dose-ranging evaluation of intravitreal siRNA PF-04523655 for diabetic macular edema (the DEGAS study). Invest Ophthalmol Vis Sci 2012. 53(12):7666-7674. [DOD] [CrossRef]
- PF-04523655 Dose Escalation Study, and Evaluation of PF-04523655 With/Without Ranibizumab in Diabetic Macular Edema (DME) (MATISSE). https://clinicaltrials.gov/ct2/show/NCT01445899, accessed May 7, 2015. [DOD]
- Napoli KL, Taylor PJ. From beach to bedside: history of the development of sirolimus. Ther Drug Monit 2001. 23(5):559-586. [DOD] [CrossRef]
- Krishnadev N, Forooghian F, Cukras C, Wong W, Saligan L, Chew EY, Nussenblatt R, Ferris F 3rd, Meyerle C. Subconjunctival sirolimus in the treatment of diabetic macular edema. Graefes Arch Clin Exp Ophthalmol 2011. 249(11):1627-1633. [DOD] [CrossRef]
- Boyer DS, Quiroz-Mercado H, Kuppermann BD, Kornfield J, Campochiaro PA, Karageozian HL, Karageozian V, Park J, Karageozian L, Kirshbaum L. Integrin peptide therapy: a new class of treatment for vascular eye diseases - the first human experience in DME. ARVO abstract 1337, 2012. [DOD]
- Multicenter clinical trial designed to evaluate the safety and exploratory efficacy of luminate (ALG-1001) as compared to avastin and focal laser photocoagulation in the treatment of diabetic macular edema. https://clinicaltrials.gov/show/ NCT02348918, accessed May 7, 2015. [DOD]
- Sun X. Synergistic anti-angiogenic effect of ATN-161 and anti-VEGF antibody in a rat model of wet age-related macular degeneration. ARVO abstract 3401, 2015. [DOD]
- Montassar F, Darche M, Elayeb M, Marrakchi N, Sennlaub F, Messadi E, Guillonneau XP. Anti-angiogenic properties of a new anti-integrin protein isolated from snake venom in mouse models of choroidal neovascularization and oxygen-induced retinopathy. ARVO abstract 50, 2015. [DOD]
- Lanza RP, Hayes JL, Chick WL. Encapsulated cell technology. Nat Biotechnol 1996. 14(9):1107-1111. [DOD] [CrossRef]
- Kontturi LS, Collin EC, Murtomäki L, Pandit AS, Yliperttula M, Urtti A. Encapsulated cells for long-term secretion of soluble VEGF receptor 1: material optimization and simulation of ocular drug response. Eur J Pharm Biopharm 2014. In press. [DOD]
- Gutierrez-Hernandez JC, Caffey S, Abdallah W, Calvillo P, Gonzalez R, Shih J, Brennan J, Zimmerman J, Martinez-Camarillo JC, Rodriguez AR, et al. One-Year Feasibility Study of Replenish MicroPump for Intravitreal Drug Delivery: A Pilot Study. Transl Vis Sci Technol. 2014. 3(4):8. [DOD]
- Humayun M, Santos A, Altamirano JC, Ribeiro R, Gonzalez R, de la Rosa A, Shih J, Pang C, Jiang F, Calvillo P, et al. Implantable MicroPump for Drug Delivery in Patients with Diabetic Macular Edema. Transl Vis Sci Technol 2014. 3(6):5. [DOD] [CrossRef]
- Preliminary safety and efficacy of the PDS-1. 0 in patients with neovascular age related macular degeneration (AMD). https://clinicaltrials.gov/ct2/show/NCT01186432, accessed May 7, 2015. [DOD]
- Silva PS, Cavallerano JD, Haddad NMN, Kwak H, Dyer KH, Omar AF, Shikari H, Aiello LM, Sun JK, Aiello LP. Peripheral lesions identified on ultrawide field imaging predict increased risk of diabetic retinopathy progression over 4 years. Ophthalmology 2015. 122(5):949-956. [DOD] [CrossRef]
- Boynton GE, Stem MS, Kwark L, Jackson GR, Farsiu S, Gardner TW. Multimodal characterization of proliferative diabetic retinopathy reveals alterations in outer retinal function and structure. Ophthalmology 2015. 122(5):957-967. [DOD] [CrossRef]
This article has been cited by other articles:

|
Efficacy of intravitreal bevacizumab combined with pan retinal photocoagulation versus panretinal photocoagulation alone in treatment of proliferative diabetic retinopathy
Sameen M, Khan MS, Mukhtar A, Yaqub MA, Ishaq M
Pak J Med Sci 2017. 33(1):142-145
|
|

|
Inhibition of Hypoxia-Induced Retinal Angiogenesis by Specnuezhenide, an Effective Constituent of Ligustrum lucidum Ait., through Suppression of the HIF-1alpha/VEGF Signaling Pathway
Wu J, Ke X, Fu W, Gao X, Zhang H, Wang W, Ma N, Zhao M, Hao X, Zhang Z
Molecules 2016. 21(12):E1756
|
|
|
Aloe-emodin suppresses hypoxia-induced retinal angiogenesis via inhibition of HIF-1alpha/VEGF pathway
Wu J, Ke X, Wang W, Zhang H, Ma N, Fu W, Zhao M, Gao X, Hao X, Zhang Z
Int J Biol Sci 2016. 12(11):1363-1371
|
|

|
Histopathological and ophthalmoscopic evaluation of apocynin on experimental proliferative vitreoretinopathy in rabbit eyes
Ozer MA, Polat N, Ozen S, Ogurel T, Parlakpinar H, Vardi N
Int Ophthalmol 2016. In press
|
|
|
Formononetin, an active compound of Astragalus membranaceus (Fisch) Bunge, inhibits hypoxia-induced retinal neovascularization via the HIF-1alpha/VEGF signaling pathway
Wu J, Ke X, Ma N, Wang W, Fu W, Zhang H, Zhao M, Gao X, Hao X, Zhang Z
Drug Des Devel Ther 2016. 10:3071-3081
|
|
|