Review
| Rev Diabet Stud,
2013,
10(2-3):157-170 |
DOI 10.1900/RDS.2013.10.157 |
Reassessing the Benefits of Statins in the Prevention of Cardiovascular Disease in Diabetic Patients - A Systematic Review and Meta-Analysis
Yu-Hung Chang1,2, Ming-Chia Hsieh2,3, Cheng-Yuan Wang4, Kun-Cheng Lin1, Yau-Jiunn Lee1
1Lee`s Endocrinology Clinic, Pingtung, 90000 Taiwan
2These authors contributed equally to this article
3Division of Endocrinology and Metabolism, Department of Internal Medicine, Changhua Christian Hospital, Taiwan
4Division of General Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung, Taiwan
Address correspondence to: Yau-Jiunn Lee, Lee’s Endocrinology Clinic, 130 Min-Tzu Rd, Pingtung, 90000 Taiwan, e-mail: t3275@ms25.hinet.net
Manuscript submitted February 9, 2013; resubmitted April 6, 2013; accepted April 30, 2013.
Keywords: cardiovascular disease, meta-analysis, dyslipidemia, LDL, lipoprotein, statin, type 2 diabetes, triglyceride
Abstract
OBJECTIVES: Despite the fact that statins have been prescribed widely, cardiovascular disease (CVD) remains the leading cause of death in diabetic patients. The aim of this study was to reassess the benefits of statins for CVD prevention in patients with diabetes mellitus. METHODS: Two independent investigators searched for prospective, randomized statin trials that investigated the power of reducing CVD in statin-treated patients. The search was performed using Pubmed, Web of Science, and CENTRAL databases. Data was extracted from eligible studies. RESULTS: A total of 7061 articles were surveyed and 22 articles were identified as eligible articles. The meta-analyses of the 22 trials showed that statin treatment was positively associated with a lowered risk of CVD in the following groups: (i) total population with pooled odds ratios (OR) of 0.791 (95 % CI: 0.74-0.846, p < 0.001), (ii) diabetic population with OR 0.792 (95% CI: 0.721-0.872, p < 0.001), and (iii) non-diabetic population with OR 0.791 (95% CI: 0.730-0.857, p < 0.001). In diabetic patients, statins were also helpful in the primary and secondary prevention of CVD, with pooled ORs of 0.757 (95% CI: 0.676 to 0.847, p < 0.001) and 0.800 (95% CI: 0.712 to 0.898, p < 0.001), respectively. However, when trials that investigated only diabetic patients (i.e., CARDS, 4D, and ASPEN) were included in the analysis, statin treatment was not found to reduce CVD significantly (OR: 0.817, 95% CI: 0.649 to 1.029, p = 0.086). Furthermore, after performing subgroup analysis, no benefit of statin treatment was found in primary prevention (OR: 0.774, 95% CI: 0.506 to 1.186, p = 0.240) or secondary prevention (OR: 0.893, 95% CI: 0.734 to 1.088, p = 0.262) of CVD in diabetic patients. CONCLUSIONS: Although our study may be limited by unmeasured confounders and heterogeneity among the studies included, the results suggest that the effects of statins in the prevention of CVD in diabetic patients are not only beneficial. More informative data are needed to verify the benefits of statins in the protection against CVD in diabetic patients.
Abbreviations: 4D - Deutsche Diabetes Dialyse Studie; 4S - Scandinavian Simvastatin Survival Study; AFCAPS/TexCAPS - Air Force/Texas Coronary Atherosclerosis Prevention Study; ALERT - Assessment of Lescol in Renal Transplantation; ALLHAT-LLT - The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial, Lipid Lowering Trial; ALLIANCE - Aggressive Lipid-Lowering Initiation Abates New Cardiac Events; ASCOT-LLA - Anglo-Scandinavian Cardiac Outcomes Trial, Lipid-Lowering Arm; ASPEN - Atorvastatin Study for the Prevention of Coronary Heart Disease Endpoints in Non-Insulin-Dependent Diabetes Mellitus; ATP III - Adult Treatment Panel III; AURORA: A study to evaluate the Use of Rosuvastatin in subjects On Regular haemodialysis: an Assessment of survival and cardiovascular events; CARDS - Collaborative Atorvastatin Diabetes Study; CARE - Cholesterol and Recurrent Events; CENTRAL - Cochrane Central Register of Controlled Trials; CI - confidence interval; CHD - coronary heart disease; CORONA - Controlled Rosuvastatin Multinational Trial in Heart Failure; CTT - Cholesterol Treatment Trialists; CVD - cardiovascular disease; df - degree of freedom; GISSI-HF - Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico-Heart Failure; GISSI-P - Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto-Prevenzione; HDL-C - high-density lipoprotein cholesterol; HMG-CoA - 3-hydroxy-3-methylglutaryl-coenzyme A; HPS - Heart Protection Study; LDL-C - low-density lipoprotein cholesterol; LIPID - Long-term Intervention with Pravastatin in Ischemic Disease; LIPS - Lescol Intervention Prevention Study; MEGA - Management of Elevated Cholesterol in the Primary Prevention Group of Adult Japanese; MeSH - Medical Subject Heading; NHANES - National Health and Nutrition Examination Survey; OR - odds ratio; PRISMA - Preferred Reporting Items for Systematic Reviews and Meta-Analyses; PROSPER - PROspective Study of Pravastatin in the Elderly at Risk; Q - Cochrane's heterogeneity statistic; SPARCL - Stroke Prevention by Aggressive Reduction in Cholesterol Levels; VADT - Veterans Affairs Diabetes Trial; WOSCOPS - West of Scotland Coronary Prevention Study
1. Introduction
Cardiovascular disease (CVD) is the leading cause of mortality and morbidity globally, in particular in patients with diabetes mellitus. Lowering serum low-density-lipoprotein cholesterol (LDL-C) level has been recommended to decrease the incidence of CVD as it decelerates the progression of atherosclerosis. The first marketed statin, named lovastatin, arrived in 1987. This drug formed a class of agents that act as HMG-CoA reductase inhibitors. It proved to be effective in reducing the LDL-C level by decreasing liver cholesterol synthesis, resulting in upregulation of LDL-C receptors and increased clearance of LDL-C. Since their discovery, statins have been widely prescribed for the purpose of reducing the risk of CVD.
In 1994, the 4S study reported that simvastatin was effective in improving the CVD risk and survival in patients with coronary heart disease (CHD) [1]. Later, the WOSCOPS study firstly demonstrated that pravastatin was helpful in the prevention of CVD occurrence in patients without CHD [2]. Subsequent trials, including AFCAPS/TexCAPS [3], CARE [4], LIPID [5], LIPS [6], HPS [7, 8], PROSPER [9], ASCOT-LLA [10, 11] and CARDS [12], showed that statins provided benefits in lowering the incidence of CVD across populations with diverse CVD risks. Although the ALLHAT-LLT [13] study reported a contrary result, and some studies also showed an inconsistent result in the diabetic subgroup [9, 10], evidence of the protection against CVD by statins, including in patients with diabetes mellitus, was soon reinforced by pivotal meta-analysis studies [14, 15]. Although subsequent 4D [16], ASPEN [17], MEGA [18] and AURORA [19] trials reported conflicting CVD results, the CTT collaboration soon consolidated the benefits of statins [20]. Based on the above evidence, professional societies have consistently recommended that statins should be the drug of choice for managing dyslipidemia in diabetic patients at risk of CVD [21, 22]. However, despite the fact that statins have been prescribed extensively over the past few decades, CVD has remained the primary cause of death in diabetic patients.
The results of previous meta-analysis reports [14, 15, 20] may be limited and should be interpreted with caution. First, from the current point of view, the studies included in this analysis may not be complete. Several recently-published reports, including CORONA [23] and SPARCL [24], have also reported the CVD outcome after statin treatment in diabetic patients. Thus, an up to date meta-analysis study that includes these publications is necessary to verify previous results. Second, most of the published literature has reported the diabetic CVD outcome by subgroup analysis, and it has been noted that the results acquired from subgroup analysis in randomized controlled trials may be biased [25]. Therefore, a meta-analysis of high-quality double blind trials that included only diabetic patients may be interesting. The aim of this study was to reassess the power of statin treatment for CVD protection in patients with diabetes mellitus.
2. Methods
This report followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statements during all stages of the design, execution and reporting when feasible [26].
2.1 Literature search
A systematic review of the available literature to the end of August 2012 was performed from the Pubmed, Web of Science and Cochrane Central Register of Controlled Trials (CENTRAL) databases by two independent investigators (Y-H Chang, M-C Hsieh). We used a combination of free key words "statin" and "cardiovascular disease", including their MeSH terms, and limited our search strategy to "Clinical Trials", "English articles" and "Human species" to find relevant articles. We also reviewed published meta-analysis reviews to identify potentially eligible studies for inclusion.
2.2 Study selection
Studies were selected if the study was prospective, randomized, compared statin with control, and reported important CVD outcomes (e.g., CVD mortality, fatal/non-fatal myocardial infarction, unstable angina, fatal/non-fatal stroke, and coronary revascularization). Articles were excluded based on the following criteria: (1) expert review articles, letters and meeting abstracts; (2) use of a surrogate as the CVD outcome (e.g., coronary angiography, computed tomography angiography, intravascular ultrasound); (3) head-to-head statin comparison; (4) high versus low statin dosage comparison; (5) non-English articles. Study quality was assessed using the Jadad scale. To resolve discrepancies, consensus was reached with other specialists (C-Y Wang, Y-J Lee) who were not involved in the initial search procedure.
2.3 Data synthesis and analysis
Data extraction was conducted independently by 2 authors (Y-H Chang, M-C Hsieh) using a standardized data extraction form. Odds ratios and 95% confidence intervals were used as the main measure of the statin effects on CVD. Information regarding the general characteristics of the study (title, publication year, study design, duration of follow-up, baseline LDL-C level, justification of early study termination, industrial funded study), characteristics of the study group (age, total participants number and proportion of diabetic patients, and definition of CVD outcome) and drug information (type of statin and dose) were also extracted. Subgroup analyses were treated as two independent studies. We contacted the original authors in cases of missing data.
In the meta-analysis graphical representation, the area of the black square indicates the weight contributed by each individual population. We estimated between-study heterogeneity using the Cochran Q statistic. Substantial heterogeneity was considered when p < 0.1, which was deemed to be a sensible cut-off value [27]. We used a random-effect model if heterogeneity was observed, while the fixed-effect model was applied in the absence of heterogeneity. In the first step of analysis, we performed a meta-analysis, including all eligible studies, followed by a subgroup analysis predefined according to diabetes status and the purpose of CVD prevention in diabetic patients. In the secondary analysis, we reassessed the effect of statins on CVD protection by including high-quality double blind randomized control trials that included only diabetic patients. In addition, we also performed sensitivity analyses by omitting one study at a time and calculating the pooled odds ratio for the remainder of the studies. Publication bias was assessed by the Egger test. All analyses were performed using Comprehensive Meta-Analysis software (Biostat, Englewood, NJ, http://www.meta-analysis.com). We considered a p-value of less than 0.05 to be statistically significant.
3. Results
The flow diagram of the selection of relevant trials for inclusion in the present systematic analysis is presented in Figure 1. A total of 7061 articles (Pubmed: 2970; Web of Science: 3720; CENTRAL: 371) were identified from the primary literature search. We screened the titles and abstracts and excluded identical articles, expert review articles, laboratory studies, non-randomized trials, and studies using surrogates as CVD endpoints, following which 25 relevant articles remained for further full-text evaluation. Three studies [28-30] were excluded after full-text review. The characteristics of the remaining 22 studies [2, 4, 6, 8, 9, 11-13, 16-19, 23, 24, 31-37] are listed in Tables 1 and 2.
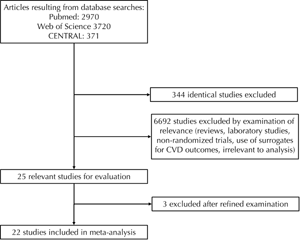 |
 |
Figure 1. Flow diagram of the selection of statin trials. |
|
Table
1.
Characteristics of the included studies (part 1) |
|
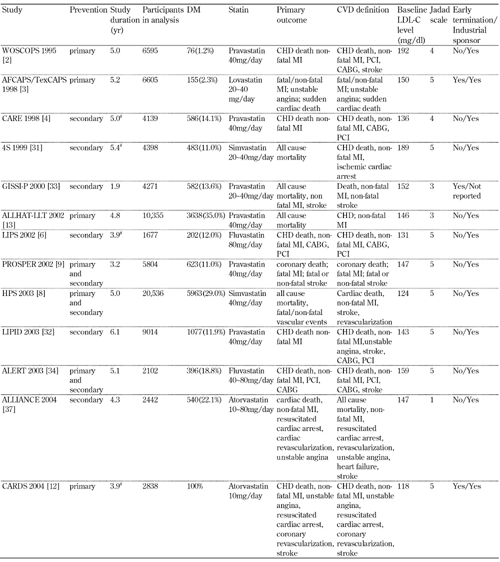 |
 |
Legend:
Baseline LDL-C levels refer to diabetic patients if available; otherwise the mean value of the overall population or the population treated with statins was reported instead. # Median. Abbreviations: DM - diabetes mellitus; CVD- cardiovascular disease; CHD - coronary heart disease; LDL-C . low-density lipoprotein cholesterol; MI - myocardial infarction; PCI - percutaneous coronary intervention; CABG - coronary artery bypass graft. |
|
Table
2.
Characteristics of the included studies (part 2) |
|
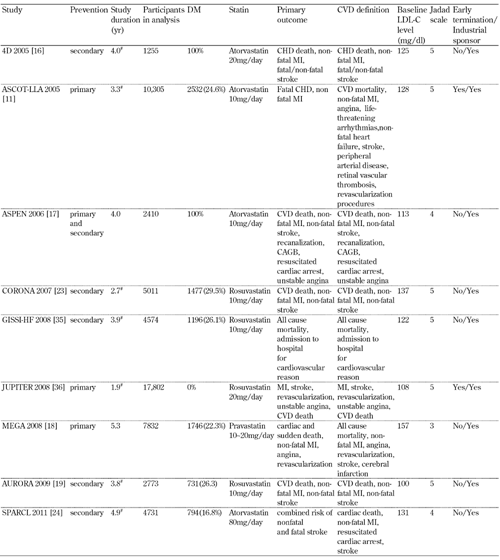 |
 |
Legend:
Baseline LDL-C levels refer to diabetic patients if available; otherwise the mean value of the overall population or the population treated with statins was reported instead. # Median. Abbreviations: DM - diabetes mellitus; CVD- cardiovascular disease; CHD - coronary heart disease; LDL-C . low-density lipoprotein cholesterol; MI - myocardial infarction; PCI - percutaneous coronary intervention; CABG - coronary artery bypass graft. |
|
3.1 Benefits of statin therapy in the protection of CVD
In Figure 2, we re-reassessed the benefits of statin treatment in CVD protection in 22 studies. In the analysis, the event number in the statin group (n = 68704) and control group (n = 68737) was 10425 and 12347, respectively. Significant heterogeneity was found (Q value = 87.88, df(Q) = 21, p < 0.001, I2 = 76.11) and the random-effect model was used for analysis. The results showed that the statin therapy significantly reduced the CVD incidence in patients with dyslipidemia, with a pooled odds ratio (OR) of 0.791 (95% CI: 0.740-0846, p < 0.001). No publication bias was found by Egger's test (p = 0.91). No studies in the individual population dramatically influenced the overall pooled odds ratio after the sensitivity test in the meta-analysis.
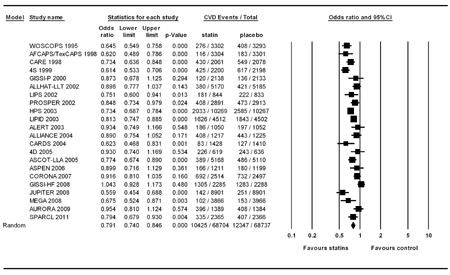 |
 |
Figure 2. Forest plot of statin treatment for CVD prevention studies in the overall population. |
|
3.2 Effect of statin on the protection of CVD differentiated by diabetes status
In Figure 3, we investigated the statin effect on CVD protection by diabetes status. In the present analysis, the WOSCOPS [2], ALERT [34], and ALLIANCE [37] studies were not included, as these trials did not specifically report the CVD outcome in diabetic patients. In the remaining 19 studies, there were 18 and 16 trials included in the analysis of diabetic patients and non-diabetic groups, respectively. For studies reporting on patients with impaired fasting blood glucose [18, 32] or metabolic syndrome [24] as a subgroup of CVD outcome, we merged these patients into the non-diabetic group. In the analysis of the diabetic group, the event number in the statin group (n = 14210) and control group (n = 14051) was 2676 and 3070, respectively. We found a significant heterogeneity (Q value = 32.29, df(Q) = 17, p = 0.014, I2 = 47.35) and used the random-effect model for analysis in the diabetic group. The results showed that statin therapy significantly reduced the CVD incidence in patients with diabetes, with a pooled OR of 0.792 (95% CI: 0.721-0.872, p < 0.001). In the analysis of the non-diabetic group, the event number in the statin group (n=48925) and control group (n = 49116) was 6879 and 8229 patients, respectively. Significant heterogeneity was also found (Q value = 60.03, df(Q) = 1, p < 0.001, I2 = 75.01) and the random-effect model was used for analysis in the non-diabetic group. The results showed that statin therapy significantly reduced the CVD incidence in non-diabetic patients, with a pooled OR of 0.791 (95% CI: 0.730-0.857, p < 0.001). No publication bias was found by Egger’s test (p = 0.56 for diabetic group; p = 0.96 for non-diabetic group). No studies in the individual population dramatically influenced the overall pooled odds ratio after sensitivity analysis was performed in the meta-analysis.
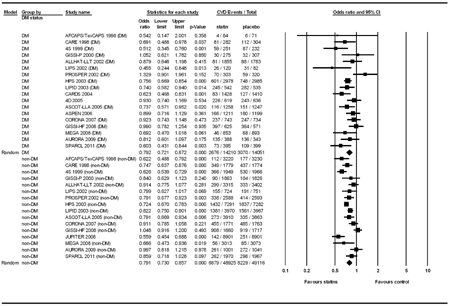 |
 |
Figure 3. Forest plot of statin treatment for CVD prevention studies stratified into diabetic and non-diabetic patients. |
|
3.3 Effect of statin on the primary and secondary prevention of CVD in diabetes
In Figure 4, we investigated the effect of statin treatment on the primary (i.e. patients without CVD history) and secondary prevention (i.e. patients with CVD history) of CVD in diabetic patients. In the analysis of primary prevention, the event number in the statin group (n = 8083) and control group (n = 8012) was 597 and 766, respectively. There was no significant heterogeneity (Q value = 10.60, df(Q) = 7, p = 0.157, I2 = 33.96) and the fixed-effect model was used for analysis. The results showed that statin therapy was effective in the primary prevention of CVD in diabetic patients, with a pooled OR of 0.757 (95% CI: 0.676-0.847, p < 0.001). In the analysis of secondary prevention, the event number in the statin group (n = 6127) and control group (n = 6039) was 2079 and 2304, respectively. There was a significant heterogeneity (Q value = 23.05, df(Q) = 12, p = 0.027, I2 = 47.95) and the random-effect model was used for analysis. The results showed that statin therapy was effective in the secondary prevention of CVD in diabetic patients, with a pooled OR of 0.800 (95% CI: 0.712-0.898, p < 0.001). No publication bias was detected by Egger’s tests in either the analysis of primary (p = 0.47) or secondary (p = 0.35) prevention. No studies in the individual population dramatically influenced the overall pooled OR after the sensitivity test in the meta-analysis.
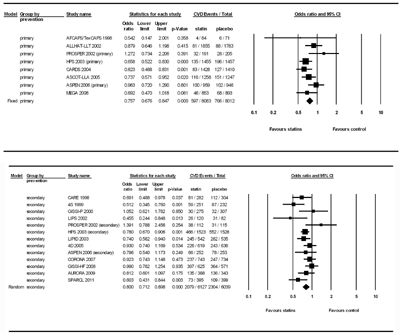 |
 |
Figure 4. Forest plot of statin therapy for CVD prevention studies in patients with diabetes mellitus stratified by primary and secondary prevention trails. |
|
3.4 Effect of statin on CVD prevention in diabetic patients - trials in diabetes only
In Figure 5, we excluded trials reporting CVD outcome in diabetic patients by subgroup analysis and included high-quality double blind randomized controlled studies with diabetic participants only. There were only 3 studies, including CARDS, 4D, and ASPEN, that fitted these criteria and remained in the analysis. While testing for the overall effect of statins on CVD protection, the event number in the statin group (n = 3258) and control group (n = 3245) was 475 and 550, respectively. There was significant heterogeneity (Q value = 5.24, df(Q) = 2, p = 0.073, I2 = 61.84) and the random-effect model was used for analysis. Surprisingly, the results showed that statin therapy was not effective in lowering the CVD incidence in diabetic patients, with a pooled OR of 0.817 (95% CI: 0.649- 1.029, p = 0.086). No publication bias was detected by Egger's test (p = 0.067). No individual study influenced the overall pooled OR after the sensitivity test in the meta-analysis. When studies were categorized by the study design for type of CVD prevention, we found no significant benefits of prescribing statins to diabetic patients, with a pooled OR of 0.774 (95% CI: 0.506-1.186, p = 0.240) by the random-effect model (Q value = 4.34, df(Q) = 1, p = 0.037, I2 = 76.97) and 0.893 (95% CI: 0.734-1.088, p = 0.262) by the fixed-effect model (Q value = 0.459, df(Q) = 1, p = 0.498, I2 = 0.00) in primary and secondary prevention, respectively.
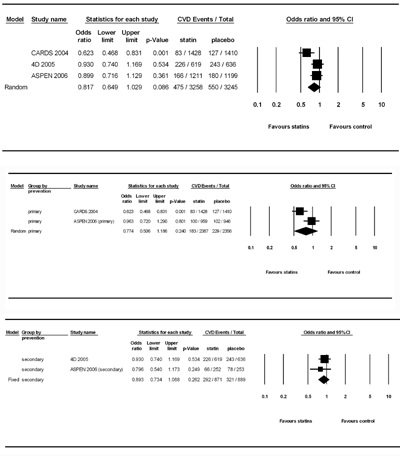 |
 |
Figure 5. Forest plot of statin therapy for CVD prevention in studies designed with double-blind, randomized controlled trials in patients with diabetes mellitus and subgroups by primary and secondary prevention trials. |
|
4. Discussion
Our results suggest that there is reasonable evidence that statins protect against CVD in diabetic and non-diabetic patients. However, further informative studies are needed to confirm that statins lower the incidence of CVD in patients with diabetes mellitus.
Since the ATP III guideline set up the LDL-C treatment target levels in patients with dyslipidemia, which are based on the CVD risk intervention according to the above mega trials [38], statins have been widely prescribed. In the NHANES survey, during 1999 to 2004, Mann et al. reported that the prescription of statins increased from 19.6% to 35.9% [39]. Meanwhile, among statins users, the prevalence of LDL-C control to the ATP III target level has increased from 49.7% to 77.6% [39]. The report clearly indicated that increased serum LDL-C was effectively lowered by statins. However, was the CVD risk reduced in parallel with the decrement of LDL-C level in patients with dyslipidemia? According to the data of NHANES acquired from 1999 to 2006, when an increased usage of lipid-lowering medication and a decreased prevalence of high LDL-C were also observed, Kuklina et al. interestingly found that the prevalence of CHD or CHD equivalents was not changed [40]. Very recently, based on data from the National Health Insurance database, which contains data from 99% of the population of Taiwan, Li et al. reported that the mortality from heart disease has significantly increased from approximately 6.5% to 10.0% in diabetic patients during the period from 2000 to 2009 in spite of the fact that statins have been widely prescribed on this island [41, 42]. Although these epidemiologic studies may lack direct evidence in questioning the CV protection provided by statins, these results may remind us to take a second look at the outcomes of these large statins trials and clinical practice guidelines on dyslipidemia management, especially in diabetic patients.
Although our pooled results supported the benefits of statins in CVD protection in accordance with previous literature [14, 15, 20], it is important to mention that the conclusion may have been biased by acquiring the CVD outcome from diabetic subgroup analysis. Subgroup analyses are often examined to test the reliability of trial conclusions across different subgroup populations defined by multiple baseline characteristics of the study participants. However, the major problem of subgroup analysis is the lack of power to detect heterogeneity in the treatment effect [43]. Furthermore, performing multiple subgroup analyses may lead to overstated and misleading results [43]. To achieve a reliable subgroup result, it has been proposed that the a priori power of subgroup analysis has to be over 40% [44]. However, the size of the subgroup population must be at least over 30% of the original study population in a study that was originally powered at 90% [44]. Among statin trials that reported CVD outcomes in the diabetic subgroup, only the ALLHAT-LLT study included over 30% of diabetic patients which provided sufficient power. Notably, the ALLHAT-LLT study was a study that did not demonstrate a CVD benefit in diabetic patients by statin therapy [13]. In association with Sun’s report that the credibility of subgroup analysis may often be overemphasized [25], it is essential to conduct an analysis that includes trials that investigated only diabetic population.
In the meta-analyses including only diabetic trials, whether investigated by overall diabetic population or the intention of CVD prevention, we surprisingly found that there was no significant benefit of statin therapy on CVD risk reduction in diabetic patients. Moreover, to relieve concerns that inclusion of the 4D study may limit our result to general diabetic patients; we removed the 4D study and reassessed our analysis. Nonetheless, an insignificant result remained with a pooled OR of 0.757 (95% CI: 0.529-1.083). Although the power issue may be a concern for interpreting these insignificant results, our results indicate that a further look into these landmark studies may be required.
For the past few years, the CARDS study has been acknowledged as a landmark study for diabetic patients [12]. The study was designed for primary CVD prevention and stopped 2 years earlier than expected. At the end of the study, CARDS showed that prescribing 10 mg of atorvastatin would reduce 37% of CVD composite events (i.e., acute coronary event, coronary revascularization and stroke) in diabetic patients aged 40-75 years who were associated with at least one CVD risk (i.e., retinopathy, albuminuria, current smoking and hypertension). According to this important reference trial, for the majority of diabetic patients, the professional society recommended that statins should be prescribed to patients who were associated with at least one CVD risk for primary CVD prevention [22]. However, results from the CARDS study should be interpreted with caution.
The first limitation of the CARDS study was the use of composite CVD endpoints. Composite endpoints are often adapted in clinical cardiovascular trials to reduce the sample size requirements and to capture the overall impact of therapeutic intervention. Although the use of composite outcomes is intriguing in facilitating the evaluation of treatment effect, it would also be regarded as ideal if the individual components contribute equally to the composite endpoint and to patients' interests [45]. However, the individual outcomes of the composite endpoint may not be regarded as biologically equivalent in the CARDS study [12, 46]. In addition, the combination of hard endpoints (i.e., fatal myocardial infarction, death from other acute coronary heart disease, fatal stroke, and resuscitated cardiac arrest) with weak endpoints (i.e., unstable angina, coronary revascularization) would cause a moderate gap of importance to patients interests [46]. Furthermore, Ferreira-Gonzalez et al. found that the relative risk was reduced substantially when a weak endpoint was added to an important one, which, as the CARDS trial did, may lead to overstating of the trial conclusion [46].
The second limitation is the early termination of the CARDS study. Bassler et al. recently found that truncated trials often demonstrate a larger treatment effect than non-truncated trials, especially those trials with an event number of less than 500, which was also true of the CARDS study [47]. There were no significant results in terms of the all-cause mortality (OR: 0.73, 95% CI: 0.52-1.01), coronary heart disease mortality (OR: 0.74, 95% CI: 0.40-1.36) and fatal stroke (OR: 0.20, 95% CI: 0.02-1.69). With similar flaws critiqued in the JUPITER study [48], the conclusion of the CARDS study remains open to discussion.
Interesting arguments were made in the 4D and ASPEN studies, which did not report positive findings in terms of CVD outcomes despite a significant reduction in LDL-C. The ASPEN study raised several possibilities (i.e. protocol changes because of changing treatment guideline, low CVD risk patients were recruited, the nature of composite endpoint) that might result in their non-significant findings and highlighted that their results do not detract from the LDL-C-lowering therapy [17]. More interestingly, the 4D study elaborated that the initiation of statin therapy may be too late for diabetic patients undergoing hemodialysis and hinted that statin therapy should be started earlier [16]. In accordance with data presented in this paper and the possible limitations of CARDS study, we suggest that there is insufficient evidence to support a role for statins in CVD protection in diabetic patients.
Two recently published articles also provide a different perception of statins. A substudy of VADT reported that the progression of coronary artery calcification was aggravated among type 2 diabetic patients with more frequent statin treatment [49]. In a cross-sectional multinational study including 6673 participants without coronary heart disease (CAD), Nakazato et al. reported that statins use was associated with a higher frequency of severe coronary artery stenosis, greater numbers of coronary vessels with obstructive CAD, and an increased presence of calcified plaque [50]. Despite the consequences of calcified plaque and CVD requiring further clarification, the results may remind us that uncertainties remain in terms of the protective effect of statins against CVD in diabetic patients.
There are limitations in our study. First, there may be concerns regarding the pooling of studies with major diversities in the study population together. Second, an inconsistent definition of CVD in these studies may also bias our results. However, these limitations may also reflect the current boundaries in examining the accuracy of these statins trials. Based on the above, a non-conflict of interest expert panel may be necessary to examine the statin trials performed in diabetic patients [51].
In conclusion, statins seems to be protective in lowering CVD risk. However, our results suggest that more informative, double blind, randomized, controlled trials are necessary to confirm the role for statins in cardiovascular protection in diabetic patients.
Disclosures: The authors report no conflict of interests.
References
- Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994. 344:1383-1389. [DOD]
- Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, MacFarlane PW, McKillop JH, Packard CJ. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med 1995. 333:1301-1307. [DOD] [CrossRef]
- Downs JR, Clearfield M, Weis S, Whitney E, Shapiro DR, Beere PA, Langendorfer A, Stein EA, Kruyer W, Gotto AM Jr. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA 1998. 279:1615-1622. [DOD] [CrossRef]
- Goldberg RB, Mellies MJ, Sacks FM, Moye LA, Howard BV, Howard WJ, Davis BR, Cole TG, Pfeffer MA, Braunwald E. Cardiovascular events and their reduction with pravastatin in diabetic and glucose-intolerant myocardial infarction survivors with average cholesterol levels: subgroup analyses in the cholesterol and recurrent events (CARE) trial. The Care Investigators. Circulation 1998. 98:2513-2519. [DOD] [CrossRef]
- Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. N Engl J Med 1998. 339:1349-1357. [DOD] [CrossRef]
- Serruys PW, de Feyter P, Macaya C, Kokott N, Puel J, Vrolix M, Branzi A, Bertolami MC, Jackson G, Strauss B, et al. Fluvastatin for prevention of cardiac events following successful first percutaneous coronary intervention: a randomized controlled trial. JAMA 2002. 287:3215-3222. [DOD] [CrossRef]
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002. 360:7-22. [DOD] [CrossRef]
- Collins R, Armitage J, Parish S, Sleigh P, Peto R, Heart Protection Study Collaborative G. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet 2003. 361:2005-2016. [DOD] [CrossRef]
- Shepherd J, Blauw GJ, Murphy MB, Bollen EL, Buckley BM, Cobbe SM, Ford I, Gaw A, Hyland M, Jukema JW, et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet 2002. 360:1623-1630. [DOD] [CrossRef]
- Sever PS, Dahlof B, Poulter NR, Wedel H, Beevers G, Caulfield M, Collins R, Kjeldsen SE, Kristinsson A, McInnes GT, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial – Lipid-Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet 2003. 361:1149-1158. [DOD] [CrossRef]
- Sever PS, Poulter NR, Dahlof B, Wedel H, Collins R, Beevers G, Caulfield M, Kjeldsen SE, Kristinsson A, McInnes GT, et al. Reduction in cardiovascular events with atorvastatin in 2,532 patients with type 2 diabetes: Anglo-Scandinavian Cardiac Outcomes Trial - Lipid-Lowering Arm (ASCOT-LLA). Diabetes Care 2005. 28:1151-1157. [DOD] [CrossRef]
- Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, Thomason MJ, Mackness MI, Charlton-Menys V, Fuller JH. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004. 364:685-696. [DOD] [CrossRef]
- ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs. usual care: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT). JAMA 2002. 288:2998-3007. [DOD] [CrossRef]
- Costa J, Borges M, David C, Vaz Carneiro A. Efficacy of lipid lowering drug treatment for diabetic and non-diabetic patients: meta-analysis of randomised controlled trials. BMJ 2006. 332:1115-1124. [DOD] [CrossRef]
- Kearney PM, Blackwell L, Collins R, Keech A, Simes J, Peto R, Armitage J, Baigent C. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet 2008. 371:117-125. [DOD] [CrossRef]
- Wanner C, Krane V, Marz W, Olschewski M, Mann JF, Ruf G, Ritz E, German D, Dialysis Study I. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med 2005. 353:238-248. [DOD] [CrossRef]
- Knopp RH, d'Emden M, Smilde JG, Pocock SJ. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: the Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in non-insulin-dependent diabetes mellitus (ASPEN). Diabetes Care 2006. 29:1478-1485. [DOD] [CrossRef]
- Tajima N, Kurata H, Nakaya N, Mizuno K, Ohashi Y, Kushiro T, Teramoto T, Uchiyama S, Nakamura H, Primary Prevention Group of Adult Japanese Study. Pravastatin reduces the risk for cardiovascular disease in Japanese hypercholesterolemic patients with impaired fasting glucose or diabetes: diabetes subanalysis of the Management of Elevated Cholesterol in the Primary Prevention Group of Adult Japanese (MEGA) Study. Atherosclerosis 2008. 199:455-462. [DOD] [CrossRef]
- Fellstrom BC, Jardine AG, Schmieder RE, Holdaas H, Bannister K, Beutler J, Chae DW, Chevaile A, Cobbe SM, Gronhagen-Riska C, et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med 2009. 360:1395-1407. [DOD] [CrossRef]
- Cholesterol Treatment Trialists' (CTT) Collaboration, Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010. 376:1670-1681. [DOD] [CrossRef]
- European Association for Cardiovascular Prevention and Rehabilitation, Reiner Z, Catapano AL, De Backer G, Graham I, Taskinen MR, Wiklund O, Agewall S, Alegria E, et al. ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J 2011. 32:1769-1818. [DOD] [CrossRef]
- American Diabetes Association. Standards of medical care in diabetes 2012. Diabetes Care 2012. 35(Suppl 1):S11-S63. [DOD]
- Kjekshus J, Apetrei E, Barrios V, Bohm M, Cleland JG, Cornel JH, Dunselman P, Fonseca C, Goudev A, Grande P, et al. Rosuvastatin in older patients with systolic heart failure. N Engl J Med 2007. 357:2248-2261. [DOD] [CrossRef]
- Callahan A, Amarenco P, Goldstein LB, Sillesen H, Messig M, Samsa GP, Altafullah I, Ledbetter LY, MacLeod MJ, Scott R, et al. Risk of stroke and cardiovascular events after ischemic stroke or transient ischemic attack in patients with type 2 diabetes or metabolic syndrome: secondary analysis of the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial. Arch Neurol 2011. 68(10):1245-1251. [DOD] [CrossRef]
- Sun X, Briel M, Busse JW, You JJ, Akl EA, Mejza F, Bala MM, Bassler D, Mertz D, Diaz-Granados N, et al. Credibility of claims of subgroup effects in randomised controlled trials: systematic review. BMJ 2012. 344:e1553. [DOD] [CrossRef]
- Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009. 151:264-269. [DOD] [CrossRef]
- Fletcher J. What is heterogeneity and is it important? BMJ 2007. 334:94-96. [DOD]
- Bradford RH, Shear CL, Chremos AN, Dujovne C, Downton M, Franklin FA, Gould AL, Hesney M, Higgins J, Hurley DP, et al. Expanded Clinical Evaluation of Lovastatin (EXCEL) study results. I. Efficacy in modifying plasma lipoproteins and adverse event profile in 8245 patients with moderate hypercholesterolemia. Arch Intern Med 1991. 151:43-49. [DOD] [CrossRef]
- Hoogwerf BJ, Waness A, Cressman M, Canner J, Campeau L, Domanski M, Geller N, Herd A, Hickey A, Hunninghake DB, et al. Effects of aggressive cholesterol lowering and low-dose anticoagulation on clinical and angiographic outcomes in patients with diabetes: the Post Coronary Artery Bypass Graft Trial. Diabetes 1999. 48:1289-1294. [DOD] [CrossRef]
- Athyros VG, Papageorgiou AA, Mercouris BR, Athyrou VV, Symeonidis AN, Basayannis EO, Demitriadis DS, Kontopoulos AG. Treatment with atorvastatin to the National Cholesterol Educational Program goal versus 'usual' care in secondary coronary heart disease prevention. The GREek Atorvastatin and Coronary-heart-disease Evaluation (GREACE) study. Curr Med Res Opin 2002. 18:220-228. [DOD] [CrossRef]
- Haffner SM, Alexander CM, Cook TJ, Boccuzzi SJ, Musliner TA, Pedersen TR, Kjekshus J, Pyorala K. Reduced coronary events in simvastatin-treated patients with coronary heart disease and diabetes or impaired fasting glucose levels: subgroup analyses in the Scandinavian Simvastatin Survival Study. Arch Intern Med 1999. 159:2661-2667. [DOD] [CrossRef]
- Keech A, Colquhoun D, Best J, Kirby A, Simes RJ, Hunt D, Hague W, Beller E, Arulchelvam M, Baker J, et al. Secondary prevention of cardiovascular events with long-term pravastatin in patients with diabetes or impaired fasting glucose: results from the LIPID trial. Diabetes Care 2003. 26:2713-2721. [DOD] [CrossRef]
- Results of the low-dose (20 mg) pravastatin GISSI Prevenzione trial in 4271 patients with recent myocardial infarction: do stopped trials contribute to overall knowledge? GISSI Prevenzione Investigators (Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico). Ital Heart J 2000. 1:810-820. [DOD]
- Holdaas H, Fellstrom B, Jardine AG, Holme I, Nyberg G, Fauchald P, Gronhagen-Riska C, Madsen S, Neumayer HH, Cole E, et al. Effect of fluvastatin on cardiac outcomes in renal transplant recipients: a multicentre, randomised, placebo-controlled trial. Lancet 2003. 361:2024-2031. [DOD] [CrossRef]
- Gissi HF, Tavazzi L, Maggioni AP, Marchioli R, Barlera S, Franzosi MG, Latini R, Lucci D, Nicolosi GL, Porcu M, et al. Effect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet 2008. 372:1231-1239. [DOD] [CrossRef]
- Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008. 359:2195-2207. [DOD] [CrossRef]
- Koren MJ, Hunninghake DB, ALLIANCE Investigators. Clinical outcomes in managed-care patients with coronary heart disease treated aggressively in lipid-lowering disease management clinics: the alliance study. J Am Coll Cardiol 2004. 44:1772-1779. [DOD]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001. 285(19):2486-2497. [DOD] [CrossRef]
- Mann D, Reynolds K, Smith D, Muntner P. Trends in statin use and low-density lipoprotein cholesterol levels among US adults: impact of the 2001 National Cholesterol Education Program guidelines. Ann Pharmacother 2008. 42:1208-1215. [DOD] [CrossRef]
- Kuklina EV, Yoon PW, Keenan NL. Trends in high levels of low-density lipoprotein cholesterol in the United States, 1999-2006. JAMA 2009. 302:2104-2110. [DOD] [CrossRef]
- Li HY, Jiang YD, Chang CH, Chung CH, Lin BJ, Chuang LM. Mortality trends in patients with diabetes in Taiwan: A nationwide survey in 2000-2009. J Formos Med Assoc 2012. 111(11):645-650. [DOD] [CrossRef]
- Lin YC, Yang CC, Chen YJ, Peng WC, Li CY, Hwu CM. Utilization of statins and aspirin among patients with diabetes and hyperlipidemia: Taiwan, 1998-2006. J Chin Med Assoc 2012. 75(11):567-572. [DOD] [CrossRef]
- Wang R, Lagakos SW, Ware JH, Hunter DJ, Drazen JM. Statistics in medicine - reporting of subgroup analyses in clinical trials. N Engl J Med 2007. 357:2189-2194. [DOD] [CrossRef]
- Wittes J. On looking at subgroups. Circulation 2009. 119:912-915. [DOD] [CrossRef]
- Montori VM, Permanyer-Miralda G, Ferreira-Gonzalez I, Busse JW, Pacheco-Huergo V, Bryant D, Alonso J, Akl EA, Domingo-Salvany A, Mills E, et al. Validity of composite end points in clinical trials. BMJ 2005. 330:594-596. [DOD] [CrossRef]
- Ferreira-Gonzalez I, Busse JW, Heels-Ansdell D, Montori VM, Akl EA, Bryant DM, Alonso-Coello P, Alonso J, Worster A, Upadhye S, et al. Problems with use of composite end points in cardiovascular trials: systematic review of randomised controlled trials. BMJ 2007. 334:786. [DOD] [CrossRef]
- Bassler D, Briel M, Montori VM, Lane M, Glasziou P, Zhou Q, Heels-Ansdell D, Walter SD, Guyatt GH, Group SS, et al. Stopping randomized trials early for benefit and estimation of treatment effects: systematic review and meta-regression analysis. JAMA 2010. 303:1180-1187. [DOD] [CrossRef]
- de Lorgeril M, Salen P, Abramson J, Dodin S, Hamazaki T, Kostucki W, Okuyama H, Pavy B, Rabaeus M. Cholesterol lowering, cardiovascular diseases, and the rosuvastatin-JUPITER controversy: a critical reappraisal. Arch Intern Med 2010. 170:1032-1036. [DOD] [CrossRef]
- Saremi A, Bahn G, Reaven PD. Progression of Vascular Calcification Is Increased With Statin Use in the Veterans Affairs Diabetes Trial (VADT). Diabetes Care 2012. 35:2390-2392. [DOD] [CrossRef]
- Nakazato R, Gransar H, Berman DS, Cheng VY, Lin FY, Achenbach S, Al-Mallah M, Budoff MJ, Cademartiri F, Callister TQ, et al. Statins use and coronary artery plaque composition: results from the International Multicenter CONFIRM Registry. Atherosclerosis 2012. 225:148-153. [DOD] [CrossRef]
- Neuman J, Korenstein D, Ross JS, Keyhani S. Prevalence of financial conflicts of interest among panel members producing clinical practice guidelines in Canada and United States: cross sectional study. BMJ 2011. 343:d5621. [DOD] [CrossRef]
This article has been cited by other articles:

|
Are diabetes guidelines truly evidence based?
Bouchonville MF, Matani S, DuBroff JJ, DuBroff RJ
Diabetes Res Clin Pract 2017. 127:70-79
|
|

|
Exploring the Management of Statin Intolerant Patients: 2016 and Beyond
Katsiki N, Athyros VG, Karagiannis A
Curr Vasc Pharmacol 2016. 14(6):523-533
|
|

|
The effect of the Conversation Map program in patients with poor glycemic control with type 2 diabetes mellitus-when usual care is not enough
Shih YT, Hsu HC, Chen SY, Hsieh CH, Wang RH, Lee YJ, Chang YH
Int J Clin Exp Med 2016. 9(2):3473-3480
|
|

|
All for Statins and Statins for All; an Update
Agouridis AP, Elisaf MS, Nair DR, Mikhailidis DP
Curr Pharm Des 2016. 22(1):18-27
|
|

|
Chromium supplementation in patients with type 2 diabetes and high risk of type 2 diabetes: a meta-analysis of randomized controlled trials
San Mauro-Martin I, Ruiz-Leon AM, Camina-Martin MA, Garicano-Vilar E, Collado-Yurrita L, Mateo-Silleras BD, Redondo Del Rio Mde P
Nutr Hosp 2016. 33(1):27
|
|

|
Genomic approach to cardiovascular disease
San Mauro-Martin I, de la Calle-de la Rosa L, Sanz-Rojo S, Garicano-Vilar E, Ciudad-Cabanas MJ, Collado-Yurrita L
Nutr Hosp 2016. 33(1):12
|
|

|
Statins and their increased risk of inducing diabetes
Agouridis AP, Kostapanos MS, Elisaf MS
Expert Opin Drug Saf 2015. 14(12):1835-144
|
|

|
A meta-analysis of the effects of statin treatment on cardiovascular events and all-cause mortality in diabetic dialysis patients
Yang M, Xie XS, Yuan WJ
Int J Clin Exp Med 2015. 8(6):8415-8424
|
|
|
The influence of strength training and fish oil supplementation on blood parameters in elderly women
de Lourdes Nahhas Rodacki C, Rodacki AL, Coelho I, Pequito D, Krause M, Bonatto S, Naliwaiko K, Fernandes LC
Rev Educ Fis 2015. 26(3):413-423
|
|

|
Influence of fish oil supplementation and strength training on some functional aspects of immune cells in healthy elderly women
de Lourdes Nahhas Rodacki C, Rodacki AL, Coelho I, Pequito D, Krause M, Bonatto S, Naliwaiko K, Fernandes LC
Br J Nutr 2015. 114(1):43-52
|
|
|
Statins' Cardiovascular Benefits Outweigh their Diabetogenicity: A Direct Comparison between Number Needed to Treat and Number Needed to Harm
Shimoyama S
Adv Pharmacoepidemiol Drug Saf 2015. 4:4
|
|
|
Beyond confusion and controversy, can we evaluate the real efficacy and safety of cholesterol-lowering with statins?
de Lorgeril M, Rabaeus M
J Controv Biomed Res 2015. 1(1):67-92
|
|
|
Efficacy of standard and intensive statin treatment for the secondary prevention of cardiovascular and cerebrovascular events in diabetes patients: a meta-analysis
de Vries FM, Kolthof J, Postma MJ, Denig P, Hak E
Plos One 2014. 9(11):e111247
|
|
|