Editorial
| Rev Diabet Stud,
2009,
6(1):6-12 |
DOI 10.1900/RDS.2009.6.6 |
The Individualized Target HbA1c: A New Method for Improving Macrovascular Risk and Glycemia Without Hypoglycemia and Weight Gain
Roy Eldor, Itamar Raz
Diabetes Research Center, Department of Medicine, Hadassah-Hebrew University Hospital, Jerusalem 91120, Israel
Address correspondence to: Roy Eldor, e-mail: eldorroy@yahoo.com
Manuscript submitted April 21, 2009; resubmitted May 17, 2009; accepted May 19, 2009.
Keywords: diabetes, cardiovascular disease, obesity, weight gain, glycemic control, ACCORD, UKPDS
Abstract
Both the DCCT and UKPDS trials demonstrated that improved glycemic control reduces microvascular complications. Inconclusive evidence, however, has remained on the question of the effect of glycemic control on macrovascular disease (with special emphasis on cardiovascular morbidity and mortality). In the last year, the data from four large trials were published, directly addressing this question (ACCORD, ADVANCE, VADT and UKPDS-80), yet the results were conflicting. Close inspection of the structure of three of these trials (ACCORD, ADVANCE and VADT) revealed inadequacies that may explain the unfavorable results, such as the inclusion of mainly elderly patients with previous macrovascular complications. It is not surprising that intensive glycemic control resulted in a rise of hypoglycemic events yet did not decrease macrovascular morbidity or mortality in these cohorts. On the other hand, the UKPDS-80 trial, a follow-up of the original UKPDS, showed that intensive glycemic control was beneficial when initiated in newly diagnosed patients. These results led us to develop a new individualized method of determining the target HbA1c based on the characteristics of the individual. This method considers the patient’s possible benefit from glycemic control, the risk of suffering hypoglycemic events and consequences suffered from the hypoglycemic event. It is essential that the target HbA1c be tailored to the patient, with different goals set for the recently diagnosed “healthy” and young patient on the one hand, and the elderly patient with co-morbidities and polypharmacy on the other hand. We further suggest a method of comparing and choosing between the different hypoglycemic drugs available. Drugs should be considered not only based on their hypoglycemic effect but also on several other attributes such as effects on weight, glycemic durability, cardiovascular protection, individual experience with the drug, method of delivery and side effect profiles. Scoring the different attributes allows us to compare between different preparations and choose the most suitable drugs for each individual patient. Using our newly suggested system, a physician will first calculate the adequate HbA1c goal for his patient and then choose the drug that will best suit him, thus tailoring the treatment to the patients needs.
Introduction
2008 was dubbed "the year of the HbA1c", with the publication of numerous large-scale trial results that were expected to shed new light on our understanding of hyperglycemic control in type 2 diabetes (T2D). However, the results provided not only a wealth of new evidence, but also conflicting and confusing information. This may cause physicians who treat diabetic patients to fail in identifying the currently perceived optimal treatment goals. In this article, we aim to clarify the data from the large clinical trials and propose a novel method for determining the glycemic treatment goal. Also, we suggest a useful new tool to achieve this goal.
HbA1c reduction in large clinical trials
If one thing was clear before 2008, it was the fact that reducing blood glucose has a beneficial effect on reducing microvascular disease. Landmark trials such as the UKPDS [1, 2], Kumamoto [3] and the DCCT [4] have proven this to be effective when implemented early in the disease course. Recent trials further stress the benefit of strict glucose control for the reduction of microvascular complications even in patients with long disease duration and multiple diabetic related complications. The recently published ADVANCE trial (Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified-Release Controlled Evaluation) included 11,140 patients with T2D and a high risk of experiencing cardiovascular events (average disease duration of 8 years), randomized to standard glucose control or intensive glucose control and followed for a median of 5 years. At the end of the follow-up period, the mean HbA1c level was 6.5% in the intensive control group and 7.3% in the standard control group. This resulted in a significant reduction in the incidence of nephropathy in the intensive control cohort (4.1% vs. 5.2%; hazard ratio, 0.79; 95% CI, 0.66 to 0.93; p = 0.006), yet with no significant effect on retinopathy (p = 0.5) [5].
Similar "signals" were seen in the VADT trial, where 1,791 military veterans (mean age 60.4 years, mean diabetes duration 11.5 years and 40% with previous cardiovascular disease) were randomly assigned to either standard or intensive glucose control. After a mean follow up period of 5.6 years, the HbA1c was 6.9% in the intensive vs. 8.4% in the standard treatment group. Although no significant reduction was observed in overall microvascular disease incidence, a nominally significant effect was detected in the progression of nephropathy (as noted by the development of albuminuria, p = 0.05) [6].
However, the results of the aforementioned trials, (with the addition of the ACCORD trial [7]) caused confusion with regards to reaching any conclusions concerning the prevention of macrovascular disease. For years it has been accepted that the higher the HbA1c, the higher the risk for developing macrovascular complications [8]. Yet the "million dollar question" of whether pharmacologically reducing the HbA1c could prevent these complications has remained unanswered. This sets the stage for the clinical trial "drama" that unfolded during 2008.
The first and most mysterious results of a large clinical trial, the ACCORD trial (Action to Control Cardiovascular Risk in Diabetes), were published in 2008. This study included 10,251 patients (mean age, 62.2 years, mean diabetes duration 10 years and 35% with previous cardiovascular disease) randomized to receive either intensive or standard glucose lowering therapy. After 1 year of therapy, the HbA1c in the intensive therapy group decreased from a mean of 8.1% to 6.4% (the bulk of this reduction occurred within the first four months after randomization, from 8.1% to 6.7%) vs. 7.5% in the standard therapy group. This was achieved by combining several hypoglycemic drug classes including metformin in 94.7%, secretagogues in 86.6%, rosiglitazone in 91.7% and insulin in 77.3% of patients. The trial was prematurely terminated after 3.5 years of follow up due to a significantly higher overall mortality noted in the intensive treatment group (257 patients in the intensive therapy group compared with 203 patients in the standard therapy group; hazard ratio, 1.22; 95% CI, 1.01 to 1.46; p = 0.04) [7]. This seemingly unexpected outcome remains unexplained. It was partially the result of an increased incidence of sudden cardiac deaths observed in the intensive treatment group. Although more severe hypoglycemic events were also seen in the intensive treatment arm, these were not statistically causally linked to the increased overall death toll.
Some months later, the ADVANCE [5] and VADT [6] trials (described earlier) were published showing no significant benefit of intensive glucose control in reducing macrovascular events. Although the number of overall deaths did not increase in the intensive treatment arm, a 117% rise in severe hypoglycemic events was seen in the VADT trial and was found to be a significant predictor of a primary outcome event (a composite of myocardial infarction, stroke, death from cardiovascular causes, congestive heart failure, surgery for vascular disease, inoperable coronary disease, and amputation for ischemic gangrene). These results were augmented by the HEART2D (Hyperglycemia and Its Effect After Acute Myocardial Infarction on Cardiovascular Outcomes in Patients With Type 2 Diabetes Mellitus) trial [9] where 1,115 diabetic patients suffering from an acute myocardial infarction, were randomly assigned to prandial or basal insulin and followed for 963 days. No difference in preventing further cardiovascular events was observed between the groups, and as a result, the trial was terminated prematurely.
Does this mean that lowering HbA1c has no benefit (or may even be harmful) in reducing cardiovascular disease? Hardly. We have known for some time that good glucose control in type 1 diabetic patients treated from the early years of their disease can reduce long term macrovascular complications. This was clearly shown in the DCCT-EDIC trial, a follow-up study of the DCCT cohort of type 1 diabetic patients. Published in 2005, the DCCT-EDIC trial demonstrated a significant reduction in the cumulative incidence of any predefined cardiovascular outcome [10]. This was despite similar glucose control in both the intensive and standard treatment groups after the original study had ended.
An analogous follow-up trial with the original UKPDS treatment cohort (termed UKPDS-80) was also published in 2008 [11]. In this trial, the UKPDS patient populations were followed for 10 years after the trials had ended. During this time, the then fairly young population had aged and the incidence of macrovascular disease had increased. In agreement with results from the DCCT-EDIC trial, the striking finding was that even though both intensive and conventional control groups had similar HbA1c levels for over 10 years after the cessation of the study, a significant reduction in the incidence of myocardial infarction in the intensive treatment group was observed. In the sulfonylurea-insulin group a 13% risk reduction for myocardial infarction (p = 0.01) and death from any cause (p = 0.007) was observed. In the metformin group, a 33% risk reduction for myocardial infarction (p = 0.005), and a 27% risk reduction for death from any cause (p = 0.002) was reported. These results are supported by the landmark STENO-2 trial, where the combination of intensive glucose, blood pressure and lipid control in high risk diabetes patients significantly reduced micro- and macrovascular complications (the hazard ratio for cardiovascular events was 0.41; 95% CI, 0.25 to 0.67; p < 0.001). The STENO-2 investigators also observed a reduction of total mortality (hazard ratio, 0.54; 95% CI, 0.32 to 0.89; p = 0.02) and death from cardiovascular causes (hazard ratio, 0.43; 95% CI, 0.19 to 0.94; p = 0.04). This finding was also published in 2008 [12].
To substantiate the evidence we must also mention the results from several trials that show a significant reduction in macrovascular complications with specific drugs designed to reduce glycemia. The Proactive trial revealed a significant secondary endpoint reduction in time to death, myocardial infarction or stroke in patients treated with pioglitazone compared with placebo [13]. Another meta-analysis of acarbose trials showed a significant reduction in myocardial infarction in patients receiving the drug [14]. Furthermore, the recently published HOME (Hyperinsulinemia: the Outcome of its Metabolic Effects) trial showed that the addition of metformin to insulin therapy in T2D patients significantly reduced macrovascular disease. A secondary endpoint aggregate comprised myocardial infarction, heart failure, changes of ECG, acute coronary syndrome, diabetic foot, stroke, transient ischemic attack, peripheral arterial disease, peripheral arterial reconstruction, PTCA, CABG, non-traumatic amputation and sudden death. The study showed beneficial effects in these endpoint elements after 4.3 years too (HR = 0.60; 95% CI, 0.40-0.92; p = 0.04; number needed to treat (NNT) = 16). This was only partially explained by a significant reduction in weight gain attributed to metformin therapy [15].
So, how do we interpret this discrepancy between the results of the recent ACCORD, VADT and ADVANCE trials on the one hand and the UKPDS-80 and similar trials on the other hand? Several important differences exist between these trials, including patient characteristics (diabetes duration, prior complications, etc.) and aggressiveness of glucose lowering. While in the UKPDS trial, the diabetic patients were relatively young, with newly diagnosed disease and without diabetes-related complications upon study entry, the patients in the ADVANCE, ACCORD and VADT studies were just the opposite. The average age of the population examined in the UKPDS-80 was 63, yet this population entered the original UKPDS clinical trial more than 15 years earlier. On the contrary, the average age in the ACCORD trial (upon entry into the treatment period) was 62 years, in VADT was 60.4 years and in ADVANCE was 66 years. The UKPDS recruited newly diagnosed patients with T2D, whereas diabetes duration upon trial entry was 10 years in the ACCORD study, 11.4 years in VADT and 8 years in ADVANCE. Up to 35% of the patients in the ACCORD trial had previous macrovascular disease, 40% in VADT and 32% in ADVANCE. Thus one may speculate that intensive glucose lowering in older patients with prolonged disease and established complications is not as beneficial as the same intervention in recently diagnosed, young and healthy diabetic patients. This is partially backed up by a sub-population analysis of the ACCORD trial. A significant risk reduction for the primary outcome was noted in the population of patients without a previous cardiovascular event (p for interaction = 0.04), and in those with an initially lower HbA1c (less than 8%, suggesting a shorter disease duration; p for interaction = 0.03).
A further consideration is that the aggressiveness of glucose lowering in these trials may have contributed to the poor outcome. In the ACCORD trial, patients were treated with combinations of 3 or 4 oral medications and insulin to achieve the target HbA1c (less than 6%) in a very short time. Similarly, in the VADT a clear change in treatment regimens from the low hypoglycemic risk medications (metformin and thiazolidinediones) to the secretagogues and insulin, was noted in order to achieve the target HbA1c in the aggressive treatment group.
In summary, we would like to quote the former president of the EASD, Prof. Ferranni (extracted from a poster presented at the recent CODHy 2008 conference): "we all are getting carried away (and often misled) by clinical 'trialism', a new epidemic among both specialists and GPs which is quickly replacing good, sensible clinical practice. For example, I can’t find anyone around here who would strenuously try to lower HbA1c below 6-6.5% in a patient 65 years of age, with long-standing diabetes and proven CVD using any mixture of SUs and insulin within an aggressive few weeks of time. Yet, this was done to thousands of patients in ACCORD."
Based on the evidence presented here, we propose a method for defining the appropriate target HbA1c for the diabetic patient in the clinic. Furthermore, we then suggest a method of achieving this target while minimally harming the patient in the process.
Suggested guideline amendment to treating hyperglycemia in diabetes
General criteria
Diabetes in connection with its diverse complications and epiphenomena is a multi-dimensional health problem. As a way out of the current confusion resulting from the partly conflicting outcomes of large clinical trials, we suggest recollecting "good clinical practice" and developing an individualized but simultaneously broadly applicable treatment method. Such a method should consider the diabetic patient as an individual who needs personalized treatment for the multi-dimensional symptomatic of diabetes and its complications. To this end, the following issues should be taken into account for the treatment of T2D patients:
-
It should be made clear that treating hyperglycemia is part of a comprehensive treatment plan tailored to the diabetic patient. Aggressive treatment of dyslipidemia using statins and lowering blood pressure are an inherent part of this plan and are clearly defined by the ADA/EASD standards of medical care guidelines [16].
-
When treating hyperglycemia in type 2 diabetes, the goals should be:
- Prevention and/or delay of subsequent micro- and macrovascular complications.
- Preservation of beta-cells.
- Optimal and physiological balance of blood glucose levels.
- Minimal side effects.
-
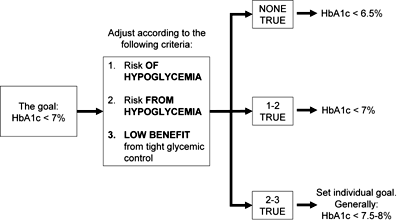
Determining the optimal HbA1c goal for the patient (Figure 1): generally the goal is HbA1c < 7%. Further adjustments are calculated based on the following criteria:
- The patient is at elevated risk of developing hypoglycemia (e.g., patients treated with secretagogues and insulin, patients prone to repeated hypoglycemic events, etc.).
- The patient is at elevated risk from hypoglycemia (e.g., elderly patients, patients after a recent cardiovascular event or other cardiac conditions that make them more prone to arrhythmia, patients at risk of falling and suffering a fracture, etc.).
- The patient will have little proven benefit from tight glucose control (e.g., lower proven benefit in patients with prolonged disease, elderly or established diabetes-related complications).
-
Using these criteria, HbA1c goals are determined as follows:
- Low risk/high benefit patients: defined as having none of the above criteria listed under 3. The HbA1c target is set at <6.5%.
- Intermediate risk/intermediate benefit patients: defined as patients having at least one of the above mentioned criteria listed under 3. The HbA1c target is determined at ≤ 7%.
- High Risk/low benefit patients: defined as having at least two of the above criteria listed under 3. The target HbA1c is individually determined in the range of 7.5-8%.
-
The treatment should be individually tailored to the patient.
 |
 |
Figure 1. Determining the optimal HbA1c goal for the patient. The figure shows the sequence of criteria according to which the treatment should be tailored to the individual patient. |
|
After setting the target HbA1c, how do we set out to achieve it? Defining specific guidelines that suggest a stepwise treatment plan sequence may not suit all patients. It may also diminish the importance of a patient-oriented treatment plan that is assembled in a dialogue between the physician and his patient. We therefore suggest an alternative method of assessing and choosing a drug treatment plan. To facilitate our recommendations, we choose to refer only to type 2 diabetic patients who are obese or overweight, since these are representative of the majority of our treated patients.
Ideal treatment of the overweight/obese patient with type 2 diabetes
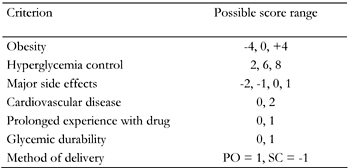
Let us imagine the ideal drug for treating hyperglycemia in the obese T2D patient. It should of course potently reduce blood sugar. Yet in 2009 this is not enough! We would also like it to reduce macrovascular complications, be weight reducing or weight neutral and preserve beta-cell mass and function (also known as glycemic durability), with no or minimal side effects. To these criteria we feel that we must add real life considerations such as whether the drug is given in an oral or subcutaneous route and whether we have prolonged experience using it. Based on these criteria we suggest a scoring system that classifies each possible treatment option (Table 1). The scoring is different for each criterion with positive numbers for a beneficial effect and negative numbers for a deleterious effect. The bulk of the score is made up of the drugs’ hypoglycemic effects, side effect profiles and effects on weight.
Table
1.
Suggested scoring system based on the "ideal hypoglycemic drug criteria" |
|
 |
 |
Legend:
Positive numbers reflect a beneficial and negative numbers a deleterious effect of the drug to be added to a therapy. Zero means no effect. The scoring system allows to compare different potential therapies when choosing the next drug to add to the therapeutic regimen of a potentially obese type 2 diabetic patient. Therefore, the most important criteria are hyperglycemia control and the drug’s effect on weight where the highest scores are allocated to. Glycemic durability as an indirect estimate of beta-cell preservation and an inherent cardiovascular protective effect are separate criteria due to their potential importance. Other considerations include the method of delivery, side effects and prolonged experience of use. PO: oral. SC: subcutaneous. |
|
We feel this scoring system reflects a change in the way we consider to choose hypoglycemic agents. Apart from their efficacy in lowering blood sugar and their side effect profile, other considerations need to be contemplated. Of these, the most important is the drug’s effect on weight. Drug-induced weight gain in the obese diabetic patient is no longer tolerated. Though little evidence exists as to the physiological effects of this weight gain, significant epidemiological data with daily clinical experience suggests this is too high a price to pay for good glucose control [17-21]. Furthermore, the glycemic durability of a drug, i.e. the length of time before another drug should be added, is also receiving more attention in building a logical treatment plan [22]. This criterion emerges as an indirect method of assessing drug effects on beta-cell mass and function.
Using this score we built a drug efficacy table that includes most of the currently available anti hyperglycemic medications (Table 2). This is a "living and breathing" table and is often changed as new evidence emerges and new drugs are made available. Furthermore, this table does not aim to determine the first, second and third choice of drug that should be used, but suggests possible alternatives that should then be tailored to the patient in a customized treatment plan. Using our drug efficacy score, pharmaceutical companies can navigate their research towards drugs that will receive higher scores and thus be more enticing as treatment options in the clinic. Also, through the treatment score table, one can learn of the current medical opinion on choosing a suitable drug for the patient. It is evident from the table that the first treatment is lifestyle changes and the first drug to consider is metformin. This coincides with current guidelines [23]. Choosing the second drug allows us to consider different drug classes such as GLP-1 analogues, DPP-4 inhibitors, acarbose, basal insulin and weight reducing medications. On the other hand, drugs such as secretagogues, previously thought to be second choice to metformin, are losing their favor due to their weight-gaining properties and side effect profiles.
Table
2.
Drug efficacy table based on the "ideal hypoglycemic drug criteria" score system |
|
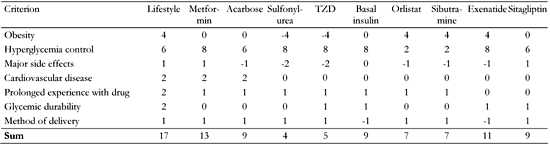 |
 |
Legend:
The final scores of the different drugs suggest possible alternatives that should be tailored to the patient in a customized treatment plan. TZD: thiazolidinediones. |
|
Conclusions
In the ever changing and dynamic world of diabetes, we are constantly refining our understanding of the disease while improving patient care and safety. In this paper, we suggest a new way of interpreting the current evidence relating to the treatment of hyperglycemia and the prevention of macrovascular disease in the diabetic patient. This will facilitate the decision-making process of the physician on establishing a treatment plan that is specifically tailored to the patient. It is not meant to be considered as a recommendation for treatment. No guideline, amendment or scoring system can replace sound clinical judgment based on an intimate knowledge and understanding of the patient. This is our belief and so should this paper be regarded.
Conflict of interest statement: The authors declare that they have no competing conflict of interests with respect to financial or other issues.
References
- Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998. 352(9131):837-853. [DOD] [CrossRef]
- Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998. 352(9131):854-865. [DOD] [CrossRef]
- Ohkubo Y, Kishikawa H, Araki E, Miyata T, Isami S, Motoyoshi S, Kojima Y, Furuyoshi N, Shichiri M. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract 1995. 28:103-117. [DOD] [CrossRef]
- The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med 1993. 329:977-986. [DOD] [CrossRef]
- ADVANCE Collaborative Group, Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008. 358(24):2560-2572. [DOD] [CrossRef]
- Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD, VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009. 360(2):129-139. [DOD] [CrossRef]
- Action to Control Cardiovascular Risk in Diabetes Study Group, Gerstein HC, Miller ME, Byington RP, Goff DC Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH Jr, Probstfield JL, Simons-Morton DG, Friedewald WT. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008. 358(24):2545-2559. [DOD] [CrossRef]
- Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 2000. 321:405-412. [DOD] [CrossRef]
- Raz I, Wilson PW, Strojek K, Kowalska I, Bozikov V, Gitt AK, Jermendy G, Campaigne BN, Kerr L, Milicevic Z, Jacober SJ. Effects of prandial versus fasting glycemia on cardiovascular outcomes in type 2 diabetes: the HEART2D trial. Diabetes Care 2009. 32:381-386. [DOD] [CrossRef]
- Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005. 353:2643-2653. [DOD] [CrossRef]
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008. 359:1577-1589. [DOD] [CrossRef]
- Rönnemaa T. Intensive glycemic control and macrovascular disease in type 2 diabetes - a report on the 44th Annual EASD meeting, Rome, Italy, September 2008. Rev Diabet Stud 2008. 5(3):180-183. [DOD] [CrossRef]
- Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 2008. 358:580-591. [DOD] [CrossRef]
- Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, Skene AM, Tan MH, Lefebvre PJ, Murray GD, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 2005. 366:1279-1289. [DOD] [CrossRef]
- Hanefeld M, Cagatay M, Petrowitsch T, Neuser D, Petzinna D, Rupp M. Acarbose reduces the risk for myocardial infarction in type 2 diabetic patients: meta-analysis of seven long-term studies. Eur Heart J 2004. 25:10-16. [DOD] [CrossRef]
- Kooy A, de Jager J, Lehert P, Bets D, Wulffele MG, Donker AJ, Stehouwer CD. Long-term effects of metformin on metabolism and microvascular and macrovascular disease in patients with type 2 diabetes mellitus. Arch Intern Med 2009. 169:616-625. [DOD] [CrossRef]
- de Valk HW. Anti-diabetic agents in type 2 diabetes: a review of new data presented and discussed on the EASD meeting in Rome, 2008. Rev Diabet Stud 2008. 5(3):184-188. [DOD] [CrossRef]
- Standards of medical care in diabetes 2008. Diabetes Care 2008. 31(Suppl 1):S12-S54. [DOD]
- Tsai CJ, Leitzmann MF, Willett WC, Giovannucci EL. Central adiposity, regional fat distribution, and the risk of cholecystectomy in women. Gut 2006. 55:708-714. [DOD] [CrossRef]
- Ogren M, Eriksson H, Bergqvist D, Sternby NH. Subcutaneous fat accumulation and BMI associated with risk for pulmonary embolism in patients with proximal deep vein thrombosis: a population study based on 23 796 consecutive autopsies. J Intern Med 2005. 258:166-171. [DOD] [CrossRef]
- Taylor EN, Stampfer MJ, Curhan GC. Obesity, weight gain, and the risk of kidney stones. JAMA 2005. 293:455-462. [DOD] [CrossRef]
- Choi HK, Atkinson K, Karlson EW, Curhan G. Obesity, weight change, hypertension, diuretic use, and risk of gout in men: the health professionals follow-up study. Arch Intern Med 2005. 165:742-748. [DOD] [CrossRef]
- Rapp K, Schroeder J, Klenk J, Stoehr S, Ulmer H, Concin H, Diem G, Oberaigner W, Weiland SK. Obesity and incidence of cancer: a large cohort study of over 145,000 adults in Austria. Br J Cancer 2005. 93:1062-1067. [DOD] [CrossRef]
- Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, Kravitz BG, Lachin JM, O'Neill MC, Zinman B, Viberti G. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 2006. 355:2427-2443. [DOD] [CrossRef]
- Nathan DM, Buse JB, Davidson MB, Ferrannini E, Holman RR, Sherwin R, Zinman B. Management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: update regarding thiazolidinediones: a consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2008. 31:173-175. [DOD] [CrossRef]
This article has been cited by other articles:

|
Personalizing treatment in type 2 diabetes: a self-monitoring of blood glucose inclusive innovative approach
Ceriello A, Gallo M, Armentano V, Perriello G, Gentile S, De Micheli A
Diabetes Technol Ther 2012. In press
|
|

|
Role of incretin therapy at various stages of diabetes
Cernea S
Rev Diabet Stud 2011. 8(3):323-338
|
|

|
Intensive glycemic control and cardiovascular disease: Are there patients who may benefit?
Chokrungvaranon N, Deer J, Reaven PD
Postgrad Med 2011. 123(6):114-123
|
|

|
The economic and clinical benefits of adequate insulin initiation and intensification in people with type 2 diabetes mellitus
Asche CV, Bode B, Busk AK, Nair S
Diabetes Obes Metab 2011. In press
|
|

|
Novel incretin-based agents and practical regimens to meet needs and treatment goals of patients with type 2 diabetes mellitus
Garber AJ
J Am Osteopath Assoc 2011. 111(7 Suppl 5):S20-S30
|
|

|
Intervention at the very early stage of type 2 diabetes
Aizawa T, Funase Y
Diabetologia 2011. 54(3):703-704
|
|

|
Diabetes and cardiovascular disease
Bloomgarden ZT
Diabetes Care 2011. 34(3):e24-30
|
|

|
Improved glycemic control without hypoglycemia in elderly diabetic patients using the ubiquitous healthcare service, a new medical information system
Lim S, Kang SM, Shin H, Lee HJ, Won Yoon J, Yu SH, Kim SY, Yoo SY, Jung HS Park KS, Ryu JO, Jang HC
Diabetes Care 2011. 34(2):308-333
|
|

|
Management of type 2 diabetes: more evidence is required to address the clinical and contextual facets
Hernandez-Jimenez S, Aguilar-Salinas CA, Mehta R, Rull JA, Gomez-Perez FJ
Curr Diabetes Rev 2011. 7(2):99-105
|
|
|
Resistance exercise training lowers HbA1c more than aerobic training in adults with type 2 diabetes
Bweir S, Al-Jarrah M, Almalty AM, Maayah M, Smirnova IV, Novikova L, Stehno-Bittel L
Diabetol Metab Syndr 2009. 1(1):27
|
|
|